Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tachimori, Shoichi*; Morita, Yasuji
Ion Exchange and Solvent Extraction, Vol.19, p.1 - 63, 2009/08
The present paper is a review of solvent extraction chemistry for reprocessing including the separation of minor actinides, cesium and strontium, prepared for the first chapter in Ion Exchange and Solvent Extraction Vol.19 which gives status of solvent extraction study in the world. The review deals with advanced PUREX process, new extractants and new extraction system for minor actinides, Cs and Sr, and etc.
Mirvaliev, R.*; Watanabe, Masayuki; Matsumura, Tatsuro; Tachimori, Shoichi*; Takeshita, Kenji*
Journal of Nuclear Science and Technology, 41(11), p.1122 - 1124, 2004/11
Times Cited Count:20 Percentile:76.55(Nuclear Science & Technology)Transmutation is a technology aimed to reduce HLW from reprocessing process. Minor actinides in the HLW will be converted to short-lived nuclides. However, lanthanides in HLW adversely affects on the efficiency of the transmutation. It is well known that separating An(III) and Ln(III) is very difficult because of their similarity of chemical properties. Therefore, the separation is one of the essential subjects to establish the transmutation technology. Considerable efforts have been devoted to the development of new extractants for the separation. N,N,N',N'-tetrakis(2-methylpyridyl)ethylenediamine (TPEN) demonstrates 100-fold preference for Am(III) over Ln(III) between stability constants with the ions in the aqueous phase. We have reported that Am(III) was selectively extracted from the aqueous phase containing Ln(III) by TPEN in nitrobenzene system and synergistic system with TPEN and D2EHPA in octanol. This work presents our recent results that Am(III) is separated from Eu(III) by a synergistic extraction system with TPEN and decanoic acid diluted with 1-octanol.
Watanabe, Masayuki; Mirvaliev, R.*; Tachimori, Shoichi; Takeshita, Kenji*; Nakano, Yoshio*; Morikawa, Koshi*; Chikazawa, Takahiro*; Mori, Ryohei*
Solvent Extraction and Ion Exchange, 22(3), p.377 - 390, 2004/06
Times Cited Count:32 Percentile:65.75(Chemistry, Multidisciplinary)The synergistic extraction with N,N,N',N'-tetrakis(2-methylpyridyl)-ethylenediamine (TPEN) and di(2-ethylhexyl)phosphoric acid (D2EHPA) demonstrates a good selectivity for Am(III) over macro amount of Ln(III) in 1-octanol. The maximum apparent separation factor, which is defined as the ratio of the distribution ratio of Am(III) to that of Eu(III), is ca. 80 while the molar fraction between D2EHPA and TPEN is 2.0. This ratio is corresponding to the result of the spectrophotometric titration, which indicates that TPEN and D2EHPA form an aggregate in 1-octanol and two D2EHPA molecules coordinate to Eu(III) TPEN complex, [Eu(TPEN)] . In the present study, the association of D2EHPA and TPEN is one of the most important factors for the synergistic extraction and the complexation of TPEN and D2EHPA with Eu(III).
. In the present study, the association of D2EHPA and TPEN is one of the most important factors for the synergistic extraction and the complexation of TPEN and D2EHPA with Eu(III).
 solutions using
solutions using  -dimethyl-
-dimethyl- -diphenylpyridine-2,6-dicarboxyamide
-diphenylpyridine-2,6-dicarboxyamideShimada, Asako; Yaita, Tsuyoshi; Narita, Hirokazu; Tachimori, Shoichi; Kimura, Takaumi; Okuno, Kenji*; *
Solvent Extraction Research and Development, Japan, 11, p.1 - 10, 2004/04
The distribution ratio ( ) of Am(III) and lighter Ln(III) in the extraction with
) of Am(III) and lighter Ln(III) in the extraction with  -dimethyl-
-dimethyl- -diphenylpyridine-2,6-dicarboxyamide(DMDPhPDA) from HNO
-diphenylpyridine-2,6-dicarboxyamide(DMDPhPDA) from HNO solutions were determined. The D's increased with an increase of HNO
solutions were determined. The D's increased with an increase of HNO concentration. Additionally, separation of Am(III) from Ln(III) were confirmed for all the HNO
concentration. Additionally, separation of Am(III) from Ln(III) were confirmed for all the HNO concentration range (S.F.=(D
concentration range (S.F.=(D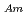 /D
/D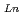 )). From 4 M HNO
)). From 4 M HNO solution with 0.5 M DMDPhPDA CHCl
solution with 0.5 M DMDPhPDA CHCl solution, the
solution, the  's of Am(III), Eu(III) and Nd(III) were 1.3, 0.25 and 0.24, respectively. This result suggests that Am(III) can be separated from Eu and Nd in the higher HNO
's of Am(III), Eu(III) and Nd(III) were 1.3, 0.25 and 0.24, respectively. This result suggests that Am(III) can be separated from Eu and Nd in the higher HNO concentration region than that has been reported so far. These
concentration region than that has been reported so far. These  's value and the S.F. were reproduced in the extraction from the metal concentration range from 10
's value and the S.F. were reproduced in the extraction from the metal concentration range from 10 M order to 10
M order to 10 M.
M.
 -dimethyl-
-dimethyl- -diphenylpyridine-2,6-dicarboxyamide(DMDPhPDA) from nitric acid solutions
-diphenylpyridine-2,6-dicarboxyamide(DMDPhPDA) from nitric acid solutionsShimada, Asako; Yaita, Tsuyoshi; Narita, Hirokazu; Tachimori, Shoichi; Okuno, Kenji*
Solvent Extraction and Ion Exchange, 22(2), p.147 - 161, 2004/03
Times Cited Count:66 Percentile:81.27(Chemistry, Multidisciplinary)The distribution ratios of lanthanides with  -dimethyl-
-dimethyl- -diphenylpyridine-2,6-dicarboxyamide(DMDPhPDA) from 1-5M nitric acid solutions were determined and the extraction mechanism was discussed on the basis of the slope analyses of acid and ligand concentration dependencies and lanthanide patterns (variation of the distribution ratio as a function of ionic radius). The lanthanide extractions were explained through two mechanisms from the standpoint of the formation of the extracted complex as follows: (1) the formation of inner-sphere complex with two DMDPhPDA molecules for light lanthanides in the extraction from HNO
-diphenylpyridine-2,6-dicarboxyamide(DMDPhPDA) from 1-5M nitric acid solutions were determined and the extraction mechanism was discussed on the basis of the slope analyses of acid and ligand concentration dependencies and lanthanide patterns (variation of the distribution ratio as a function of ionic radius). The lanthanide extractions were explained through two mechanisms from the standpoint of the formation of the extracted complex as follows: (1) the formation of inner-sphere complex with two DMDPhPDA molecules for light lanthanides in the extraction from HNO less than 3M, and (2) the formation of outer-sphere complex with the third DMDPhPDA molecules in addition to the inner-sphere complex in the extraction of light lanthanides from HNO
less than 3M, and (2) the formation of outer-sphere complex with the third DMDPhPDA molecules in addition to the inner-sphere complex in the extraction of light lanthanides from HNO more than 3M, and those of heavy lanthanides from 1-5M HNO
more than 3M, and those of heavy lanthanides from 1-5M HNO solutions. Nitric acid concentration is more influential than the ligand concentration in the formation of outer-sphere complex.
solutions. Nitric acid concentration is more influential than the ligand concentration in the formation of outer-sphere complex.
Watanabe, Masayuki; Nankawa, Takuya*; Yamada, Teppei*; Kimura, Takaumi; Namiki, Kosuke*; Murata, Masaki*; Nishihara, Hiroshi*; Tachimori, Shoichi
Inorganic Chemistry, 42(22), p.6977 - 6979, 2003/11
Times Cited Count:34 Percentile:75.29(Chemistry, Inorganic & Nuclear)A tripodal ligand, tris(2-pyridyl)carbinol affords a novel tetradentate coordination mode in homodinuclear lanthanide complexes, which exhibit remarkably short distance between the metal ions. Strong fluorescence of Eu(III) and Tb(III) complexes with the ligand demonstrate that the ligand has a suitable excited state for energy transfer from the ligand to Eu(III) and Tb(III) center.
Assefa, Z.*; Yaita, Tsuyoshi; Haire, R. G.*; Tachimori, Shoichi
Inorganic Chemistry, 42(23), p.7375 - 7377, 2003/11
Times Cited Count:11 Percentile:36.73(Chemistry, Inorganic & Nuclear)The 6-methyl- 2-(2-pyridyl)-benzimidazole (biz) ligand coordinates with the actinide species in solution, and the complexes display efficient intra-molecular energy-transfer processes. The energy transfer in the Cm(III):biz system proceeds in a non-radiative mode, whereas a radiative mode is the principal mechanism in the Am(III)-biz system.
Tachimori, Shoichi; Suzuki, Shinichi; Sasaki, Yuji; Apichaibukol, A.
Solvent Extraction and Ion Exchange, 21(5), p.707 - 715, 2003/09
Times Cited Count:48 Percentile:75.63(Chemistry, Multidisciplinary)no abstracts in English
 (pyridine-dicarboxy-amide)
(pyridine-dicarboxy-amide) complexes
complexesDobler, M.; Hirata, Masaru; Tachimori, Shoichi
Physical Chemistry Chemical Physics, 2003(5), p.2499 - 2504, 2003/05
no abstracts in English
Suzuki, Hideya*; Naganawa, Hirochika; Tachimori, Shoichi
Solvent Extraction and Ion Exchange, 21(4), p.527 - 546, 2003/03
Times Cited Count:12 Percentile:45.03(Chemistry, Multidisciplinary)no abstracts in English
 and UO
and UO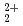 ions with tridentate ligand diglycolamide (DGA)
ions with tridentate ligand diglycolamide (DGA)Hirata, Masaru; Guilbard, P.*; Dobler, M.; Tachimori, Shoichi
Physical Chemistry Chemical Physics, 5(4), p.691 - 695, 2003/02
Times Cited Count:20 Percentile:54.61(Chemistry, Physical)no abstracts in English
Sasaki, Yuji; Suzuki, Shinichi; Tachimori, Shoichi*; Kimura, Takaumi
Proceedings of GLOBAL2003 Atoms for Prosperity; Updating Eisenhower's Global Vision for Nuclear Energy (CD-ROM), 4 Pages, 2003/00
no abstracts in English
Watanabe, Masayuki; Mirvaliev, R.*; Tachimori, Shoichi; Takeshita, Kenji*; Nakano, Yoshio*; Morikawa, Koshi*; Mori, Ryohei*
Chemistry Letters, 31(12), p.1230 - 1231, 2002/12
Times Cited Count:40 Percentile:73.3(Chemistry, Multidisciplinary)Encapsulative semi-podand type ligand, N, N, N', N' #8211; tetrakis(2-methylpyridyl)ethylenediamine (TPEN) gives good selectivity of Am(III) over trivalent lanthanide by solvent extraction. This is the first robust extraction system which can separate Am(III) from trivalent lanthanide.
Morita, Yasuji; Tachimori, Shoichi; Koma, Yoshikazu*; Aoshima, Atsushi*
JAERI-Research 2002-017, 20 Pages, 2002/08
The present report describes the results of a joint study between Japan Nuclear Cycle Development Institute (JNC) and Japan Atomic Energy Research Institute (JAERI) on actinide separation process from high-level liquid waste. The purpose of the joint study is to point out common subjects in process development by an overall evaluation of each actinide separation process: TRUEX/SETFICS Process studied in JNC and DIDPA Extraction Process studied in JAERI. The result of the evaluation showed that both processes have common subjects to be studied in sub-processes such as treatment step for spent solvent or DTPA waste solution and solvent washing step for recycling, although the main process is different from each other. It is necessary to develop the sub-processes and to test the whole process including the sub-processes. Two essential requirements: the cost reduction and the minimization of secondary wastes, are very important in future research and development for more rational and effective actinide separation process.
Sugo, Yumi; Sasaki, Yuji; Tachimori, Shoichi
Radiochimica Acta, 90(3), p.161 - 165, 2002/04
Times Cited Count:153 Percentile:99.24(Chemistry, Inorganic & Nuclear)no abstracts in English
Sasaki, Yuji; Tachimori, Shoichi
Solvent Extraction and Ion Exchange, 20(1), p.21 - 34, 2002/01
Times Cited Count:186 Percentile:95.52(Chemistry, Multidisciplinary)no abstracts in English
Tachimori, Shoichi; Suzuki, Shinichi; Sasaki, Yuji
Nihon Genshiryoku Gakkai-Shi, 43(12), p.1235 - 1241, 2001/12
Times Cited Count:29 Percentile:87.17(Nuclear Science & Technology)no abstracts in English
Tachimori, Shoichi
JAERI-Research 2001-048, 23 Pages, 2001/10
A new chemical process, ARTIST process, is proposed for the treatment of spent nuclear fuel. The main concept of the ARTIST process is to recover and stock all actinides (Ans) in two groups, uranium (U) and a mixture of transuranics (TRU), to preserve their resource value and to dispose solely fission products (FPs). The process composed of two main steps, an U exclusive isolation and a total recovery of TRU; which copes with the nuclear non-proliferation measures, and additionally Pu separation process and soft N-donor process if requested, and optionally processes for separation of long-lived FPs. These An products: U-product and TRU-product, are to be solidified by calcination and allowed to the interim stockpile for future utilization. These separations are achieved by use of amidic extractants in accord with the CHON principle. The technical feasibility of the ARTIST process was explained by the performance of both the branched-alkyl monoamides the diglycolic amide (TODGA) in thorough extraction of all TRU by tridentate fashon.
Morita, Yasuji; Sasaki, Yuji; Tachimori, Shoichi
Proceedings of International Conference on Back-End of the Fuel Cycle: From Research to Solutions (GLOBAL 2001) (CD-ROM), 7 Pages, 2001/09
no abstracts in English
Naganawa, Hirochika; Suzuki, Hideya; Tachimori, Shoichi; Nasu, Akinobu*; Sekine, Tatsuya*
Physical Chemistry Chemical Physics, 3(12), p.2509 - 2517, 2001/06
Times Cited Count:34 Percentile:70.77(Chemistry, Physical)no abstracts in English