Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
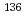 Xe ions on proton, deuteron, and carbon targets
Xe ions on proton, deuteron, and carbon targetsSun, X. H.*; Wang, H.*; Otsu, Hideaki*; Sakurai, Hiroyoshi*; Ahn, D. S.*; Aikawa, Masayuki*; Fukuda, Naoki*; Isobe, Tadaaki*; Kawakami, Shunsuke*; Koyama, Shumpei*; et al.
Physical Review C, 101(6), p.064623_1 - 064623_12, 2020/06
Times Cited Count:5 Percentile:51.18(Physics, Nuclear)The spallation and fragmentation reactions of 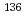 Xe induced by proton, deuteron and carbon at 168 MeV/nucleon were studied at RIKEN Radioactive Isotope Beam Factory via the inverse kinematics technique. The cross sections of the lighter products are larger in the carbon-induced reactions due to the higher total kinetic energy of carbon. The energy dependence was investigated by comparing the newly obtained data with previous results obtained at higher reaction energies. The experimental data were compared with the results of SPACS, EPAX, PHITS and DEURACS calculations. These data serve as benchmarks for the model calculations.
Xe induced by proton, deuteron and carbon at 168 MeV/nucleon were studied at RIKEN Radioactive Isotope Beam Factory via the inverse kinematics technique. The cross sections of the lighter products are larger in the carbon-induced reactions due to the higher total kinetic energy of carbon. The energy dependence was investigated by comparing the newly obtained data with previous results obtained at higher reaction energies. The experimental data were compared with the results of SPACS, EPAX, PHITS and DEURACS calculations. These data serve as benchmarks for the model calculations.
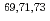 Co
CoLokotko, T.*; Leblond, S.*; Lee, J.*; Doornenbal, P.*; Obertelli, A.*; Poves, A.*; Nowacki, F.*; Ogata, Kazuyuki*; Yoshida, Kazuki; Authelet, G.*; et al.
Physical Review C, 101(3), p.034314_1 - 034314_7, 2020/03
Times Cited Count:10 Percentile:75.41(Physics, Nuclear)The structures of the neutron-rich 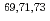 Co isotopes were investigated via (
Co isotopes were investigated via (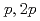 ) knockout reactions at the Radioactive Isotope Beam Factory, RIKEN. Level schemes were reconstructed using the
) knockout reactions at the Radioactive Isotope Beam Factory, RIKEN. Level schemes were reconstructed using the 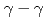 coincidence technique, with tentative spin-parity assignments based on the measured inclusive and exclusive cross sections. Comparison with shell-model calculations suggests coexistence of spherical and deformed shapes at low excitation energies in the
coincidence technique, with tentative spin-parity assignments based on the measured inclusive and exclusive cross sections. Comparison with shell-model calculations suggests coexistence of spherical and deformed shapes at low excitation energies in the 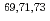 Co isotopes.
Co isotopes.
 Ni from the (
Ni from the ( ,
,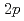 ) reaction
) reactionElekes, Z.*; Kripk ,
,  *; Sohler, D.*; Sieja, K.*; Ogata, Kazuyuki*; Yoshida, Kazuki; Doornenbal, P.*; Obertelli, A.*; Authelet, G.*; Baba, Hidetada*; et al.
*; Sohler, D.*; Sieja, K.*; Ogata, Kazuyuki*; Yoshida, Kazuki; Doornenbal, P.*; Obertelli, A.*; Authelet, G.*; Baba, Hidetada*; et al.
Physical Review C, 99(1), p.014312_1 - 014312_7, 2019/01
Times Cited Count:11 Percentile:73.49(Physics, Nuclear)The nuclear structure of the 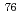 Ni nucleus was investigated by (
Ni nucleus was investigated by ( ,
,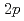 ) reaction using a NaI(Tl) array to detect the deexciting prompt
) reaction using a NaI(Tl) array to detect the deexciting prompt  rays. A new transition with an energy of 2227 keV was identified by
rays. A new transition with an energy of 2227 keV was identified by 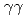 and
and 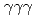 coincidences. Our shell-model calculations using the Lenzi, Nowacki, Poves, and Sieja interaction produced good candidates for the experimental proton hole states in the observed energy region, and the theoretical cross sections showed good agreement with the experimental values. Although we could not assign all the experimental states to the theoretical ones unambiguously, the results are consistent with a reasonably large Z = 28 shell gap for nickel isotopes in accordance with previous studies.
coincidences. Our shell-model calculations using the Lenzi, Nowacki, Poves, and Sieja interaction produced good candidates for the experimental proton hole states in the observed energy region, and the theoretical cross sections showed good agreement with the experimental values. Although we could not assign all the experimental states to the theoretical ones unambiguously, the results are consistent with a reasonably large Z = 28 shell gap for nickel isotopes in accordance with previous studies.
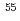 Sc and development of the
Sc and development of the 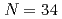 subshell closure
subshell closureSteppenbeck, D.*; Takeuchi, Satoshi*; Aoi, Nori*; Doornenbal, P.*; Matsushita, Masafumi*; Wang, H.*; Baba, Hidetada*; Go, Shintaro*; Holt, J. D.*; Lee, J.*; et al.
Physical Review C, 96(6), p.064310_1 - 064310_10, 2017/12
Times Cited Count:18 Percentile:80.37(Physics, Nuclear)no abstracts in English
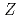 =28 and
=28 and  =50; Spectroscopy of
=50; Spectroscopy of 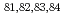 Zn
ZnShand, C. M.*; Podoly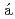 k, Zs.*; G
k, Zs.*; G rska, M.*; Doornenbal, P.*; Obertelli, A.*; Nowacki, F.*; Otsuka, T.*; Sieja, K.*; Tostevin, J. A.*; Tsunoda, T.*; et al.
rska, M.*; Doornenbal, P.*; Obertelli, A.*; Nowacki, F.*; Otsuka, T.*; Sieja, K.*; Tostevin, J. A.*; Tsunoda, T.*; et al.
Physics Letters B, 773, p.492 - 497, 2017/10
Times Cited Count:25 Percentile:87.67(Astronomy & Astrophysics) Kr
KrFlavigny, F.*; Doornenbal, P.*; Obertelli, A.*; Delaroche, J.-P.*; Girod, M.*; Libert, J.*; Rodriguez, T. R.*; Authelet, G.*; Baba, Hidetada*; Calvet, D.*; et al.
Physical Review Letters, 118(24), p.242501_1 - 242501_6, 2017/06
Times Cited Count:38 Percentile:87.16(Physics, Multidisciplinary) decay of unbound neutron-hole states in
decay of unbound neutron-hole states in  Sn
SnVaquero, V.*; Jungclaus, A.*; Doornenbal, P.*; Wimmer, K.*; Gargano, A.*; Tostevin, J. A.*; Chen, S.*; N cher, E.*; Sahin, E.*; Shiga, Yoshiaki*; et al.
cher, E.*; Sahin, E.*; Shiga, Yoshiaki*; et al.
Physical Review Letters, 118(20), p.202502_1 - 202502_5, 2017/05
Times Cited Count:21 Percentile:76.72(Physics, Multidisciplinary)Chen, S.*; Doornenbal, P.*; Obertelli, A.*; Rodriguez, T. R.*; Authelet, G.*; Baba, Hidetada*; Calvet, D.*; Ch teau, F.*; Corsi, A.*; Delbart, A.*; et al.
teau, F.*; Corsi, A.*; Delbart, A.*; et al.
Physical Review C, 95(4), p.041302_1 - 041302_6, 2017/04
Times Cited Count:26 Percentile:88.25(Physics, Nuclear)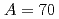 isobars from the
isobars from the 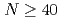 island of inversion
island of inversionMorales, A. I.*; Benzoni, G.*; Watanabe, H.*; Tsunoda, Yusuke*; Otsuka, T.*; Nishimura, Shunji*; Browne, F.*; Daido, R.*; Doornenbal, P.*; Fang, Y.*; et al.
Physics Letters B, 765, p.328 - 333, 2017/02
Times Cited Count:33 Percentile:92.03(Astronomy & Astrophysics)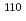 Zr
ZrPaul, N.*; Corsi, A.*; Obertelli, A.*; Doornenbal, P.*; Authelet, G.*; Baba, Hidetada*; Bally, B.*; Bender, M.*; Calvet, D.*; Ch teau, F.*; et al.
teau, F.*; et al.
Physical Review Letters, 118(3), p.032501_1 - 032501_7, 2017/01
Times Cited Count:44 Percentile:89.34(Physics, Multidisciplinary)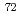 Ni
NiMorales, A. I.*; Benzoni, G.*; Watanabe, H.*; Nishimura, Shunji*; Browne, F.*; Daido, R.*; Doornenbal, P.*; Fang, Y.*; Lorusso, G.*; Patel, Z.*; et al.
Physical Review C, 93(3), p.034328_1 - 034328_14, 2016/03
Times Cited Count:25 Percentile:84.68(Physics, Nuclear)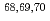 Mn; Probing collectivity up to N=44 in Fe isotopic chain
Mn; Probing collectivity up to N=44 in Fe isotopic chainBenzoni, G.*; Morales, A. I.*; Watanabe, H.*; Nishimura, Shunji*; Coraggio, L.*; Itaco, N.*; Gargano, A.*; Browne, F.*; Daido, R.*; Doornenbal, P.*; et al.
Physics Letters B, 751, p.107 - 112, 2015/12
Times Cited Count:20 Percentile:77.61(Astronomy & Astrophysics)Iwai, Yasunori; Kubo, Hitoshi*; Oshima, Yusuke*; Noguchi, Hiroshi*; Edao, Yuki; Taniuchi, Junichi*
Fusion Science and Technology, 68(3), p.596 - 600, 2015/10
Times Cited Count:2 Percentile:17.42(Nuclear Science & Technology)We have newly developed the hydrophobic platinum honeycomb catalysts applicable to tritium oxidation reactor since the honeycomb-shape catalyst can decrease the pressure drop. Two types of hydrophobic honeycomb catalyst have been test-manufactured. One is the hydrophobic platinum catalyst on a metal honeycomb. The other is the hydrophobic platinum catalyst on a ceramic honeycomb made of silicon carbide. The fine platinum particles around a few nanometers significantly improve the catalytic activity for the oxidation tritium at a tracer concentration. The hydrogen concentration in the gaseous feed slightly affects the overall reaction rate constant for hydrogen oxidation. Due to the competitive adsorption of hydrogen and water molecules on platinum surface, the overall reaction rate constant has the bottom value. The hydrogen concentration for the bottom value is 100 ppm under the dry feed gas. We have experimentally confirmed the activity of these honeycomb catalysts is as good as that of pellet-shape hydrophobic catalyst. The results support the hydrophobic honeycomb catalysts are applicable to tritium oxidation reactor.
 Ar and the
Ar and the  =32 subshell closure
=32 subshell closureSteppenbeck, D.*; Takeuchi, Satoshi*; Aoi, Nori*; Doornenbal, P.*; Matsushita, Masafumi*; Wang, H.*; Utsuno, Yutaka; Baba, Hidetada*; Go, Shintaro*; Lee, J.*; et al.
Physical Review Letters, 114(25), p.252501_1 - 252501_6, 2015/06
Times Cited Count:45 Percentile:87.92(Physics, Multidisciplinary)The neutron-rich nucleus  Ar is produced by the fragmentation reactions of
Ar is produced by the fragmentation reactions of 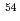 Ca,
Ca, 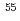 Sc, and
Sc, and 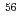 Ti at the RIBF facility in RIKEN, and its deexcited
Ti at the RIBF facility in RIKEN, and its deexcited  rays are observed for the first time. The first
rays are observed for the first time. The first 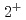 level in
level in  Ar is identified to lie at 1178(18)keV from the most intense
Ar is identified to lie at 1178(18)keV from the most intense  -ray spectra. This experimental data, together with the systematics of the
-ray spectra. This experimental data, together with the systematics of the 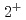 levels for surrounding nuclei, is analyzed with large-scale shell-model calculations. Consequently, the
levels for surrounding nuclei, is analyzed with large-scale shell-model calculations. Consequently, the 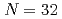 sub-shell gap in
sub-shell gap in  Ar is equivalent to that of
Ar is equivalent to that of 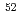 Ca, thus making the
Ca, thus making the 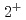 level in
level in  Ar higher than that of
Ar higher than that of  Ar. The shell-model calculation also predicts that the
Ar. The shell-model calculation also predicts that the 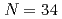 sub-shell gap enhances in going from Ca to Ar, which will be verified by forthcoming experiments for
sub-shell gap enhances in going from Ca to Ar, which will be verified by forthcoming experiments for 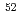 Ar.
Ar.
Iwai, Yasunori; Kubo, Hitoshi*; Sato, Katsumi; Oshima, Yusuke*; Noguchi, Hiroshi*; Taniuchi, Junichi*
Proceedings of 7th Tokyo Conference on Advanced Catalytic Science and Technology (TOCAT-7) (USB Flash Drive), 2 Pages, 2014/06
Hydrophobic platinum catalysts have been developed especially for combustion of hydrogen isotopes released in a nuclear facility. A new type of hydrophobic hydrogen combustion catalyst commercially named TKK-KNOITS catalyst is hardly susceptible to water mist and water vapor in the atmosphere and water produced by hydrogen combustion. It is capable of maintaining the activity even at relatively low temperatures. The TKK-KNOITS catalyst is superior to other previous hydrophobic catalysts in applicability to wide range of hydrogen concentration from very thin to dense. The catalyst which carrier is composed of inorganic oxide has thermal stability up to 873 K.
 Ca
CaSteppenbeck, D.*; Takeuchi, Satoshi*; Aoi, Nori*; Doornenbal, P.*; Matsushita, Masafumi*; Wang, H.*; Baba, Hidetada*; Fukuda, Naoki*; Go, Shintaro*; Homma, Michio*; et al.
Nature, 502(7470), p.207 - 210, 2013/10
Times Cited Count:286 Percentile:99.78(Multidisciplinary Sciences)no abstracts in English
Iwai, Yasunori; Sato, Katsumi; Taniuchi, Junichi*; Noguchi, Hiroshi*; Kubo, Hitoshi*; Harada, Nobuo*; Oshima, Yusuke*; Yamanishi, Toshihiko
Journal of Nuclear Science and Technology, 48(8), p.1184 - 1192, 2011/08
Times Cited Count:34 Percentile:91.73(Nuclear Science & Technology)The inorganic-based hydrophobic Pt-catalyst named H1P has been developed especially for efficient oxidation of a tracer level of tritium in the ambient temperature range even in the presence of saturated water vapor. The overall reaction rate constant for H1P catalyst in the ambient temperature range was considerably larger than that for traditionally applied Pt/Al O
O catalyst. Moreover, the decrease in reaction rate for H1P in the presence of saturated water vapor compared with in the absence of water vapor was slight due to its excellence in hydrophobic performance. Oxidation reaction on the catalyst surface is the rate-controlling step in the ambient temperature range and diffusion in a catalyst substratum above 313 K due to its fine porosity. The overall reaction rate constant in the ambient temperature range was dependent on the space velocity and hydrogen concentration in carrier.
catalyst. Moreover, the decrease in reaction rate for H1P in the presence of saturated water vapor compared with in the absence of water vapor was slight due to its excellence in hydrophobic performance. Oxidation reaction on the catalyst surface is the rate-controlling step in the ambient temperature range and diffusion in a catalyst substratum above 313 K due to its fine porosity. The overall reaction rate constant in the ambient temperature range was dependent on the space velocity and hydrogen concentration in carrier.
Iwai, Yasunori; Sato, Katsumi; Kubo, Hitoshi*; Oshima, Yusuke*; Noguchi, Hiroshi*; Taniuchi, Junichi*
no journal, ,
Taking an accident such as loss of electric power into consideration, a hydrophobic catalyst, over which tritium and oxygen can react efficiently at room temperature, will contribute greatly to the fusion safety. Japan Atomic Energy Agency and Tanaka Kikinzoku Kogyo K.K. has jointly developed the hydrophobic platinum catalyst TKK-KNOITS for the oxidation of very thin tritium at room temperature. The overall reaction rate constant at room temperature for TKK-KNOITS was much larger than that for TKK-H1P, previously reported. The volume of catalytic reactor can be significantly reduced when the reactor is packed with TKK-KNOITS.
Iwai, Yasunori; Sato, Katsumi; Yamanishi, Toshihiko; Taniuchi, Junichi*; Noguchi, Hiroshi*; Kubo, Hitoshi*; Harada, Nobuo*; Oshima, Yusuke*
no journal, ,
The inorganic-based hydrophobic Pt-catalyst named H1P has been developed especially for efficient oxidation of a tracer level of tritium at room temperature even in the presence of saturated water vapor. Overall reaction rate constant of tritium oxidation on a H1P catalyst in a flow-through system were determined as a function of space velocity, hydrogen concentration in carrier, temperature of catalyst, water vapor concentration in carrier. The overall reaction rate constant for H1P catalyst at room temperature was considerably larger than that for traditionally applied Pt-alumina catalyst. The overall reaction rate constant at room temperature was dependent on the space velocity and hydrogen concentration in carrier. The overall reaction rate constant under the dry and wet conditions was proportional to hydrogen concentration in carrier to the power 0.33 and 0.44, respectively.
Kubo, Hitoshi*; Oshima, Yusuke*; Noguchi, Hiroshi*; Taniuchi, Junichi*; Sato, Katsumi; Iwai, Yasunori
no journal, ,
Regarding the fusion plants, radioactive tritium is a recyclable fuel. Tritium release into the environment should be controlled as low as possible even in case of a severe accident. In a severe accident in a light water reactor accompanied with loss of electricity, serious damage of the containment and safety-relevant components by detonation of the hydrogen-air mixture should be prevented. Oxidation of hydrogen in a catalytic reactor is considered a reliable way to remove hydrogen and tritium. The typical hydrophilic catalysts are useless due to the loss of activity by exposure to moisture at room temperature. The existing polymer-based hydrophobic catalysts have disadvantage of burnable. In the presentation, fabrication technique for inorganic-based hydrophobic catalysts to achieve a good balance of thermal stability and oxidation activity at room temperature is discussed.