Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Reactions to Achieve Mass-spectrometric Discrimination in Simultaneous Plutonium-isotope Speciation with Inductively Coupled Plasma-Tandem Mass Spectrometry
Reactions to Achieve Mass-spectrometric Discrimination in Simultaneous Plutonium-isotope Speciation with Inductively Coupled Plasma-Tandem Mass SpectrometryMatsueda, Makoto; Kawakami, Tomohiko*; Koarai, Kazuma; Terashima, Motoki; Fujiwara, Kenso; Iijima, Kazuki; Furukawa, Makoto*; Takagai, Yoshitaka*
Chemistry Letters, 51(7), p.678 - 682, 2022/07
Times Cited Count:5 Percentile:61.39(Chemistry, Multidisciplinary)New methodology for a simultaneous isotope speciation of various Pu isotopes without complicated isobaric interferences is developed by using inductively coupled plasma-mass spectrometry (ICP-MS). In analyzing ICP tandem MS (ICP-MS/MS), CO gas reactions in a dynamic reaction cell (DRC) almost eliminated the background noise intensity produced by isobaric interference from isotopes originating from actinides such as Am, Cm, and U at the locations (m/z) of significant Pu isotopes (
gas reactions in a dynamic reaction cell (DRC) almost eliminated the background noise intensity produced by isobaric interference from isotopes originating from actinides such as Am, Cm, and U at the locations (m/z) of significant Pu isotopes ( Pu,
Pu,  Pu,
Pu,  Pu,
Pu, 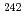 Pu,
Pu,  Pu).
Pu).
Tachi, Yukio; Sato, Tomofumi*; Akagi, Yosuke*; Kawamura, Makoto*; Nakane, Hideji*; Terashima, Motoki; Fujiwara, Kenso; Iijima, Kazuki
Science of the Total Environment, 724, p.138098_1 - 138098_11, 2020/07
Times Cited Count:13 Percentile:57.17(Environmental Sciences)To understand and predict radiocesium transport behaviors in the environment, highly contaminated sediments from Ukedo and Odaka rivers around the Fukushima Daiichi Nuclear Power Plant were investigated systematically focusing on key factors controlling radiocesium sorption and fixation, including particle size, clay mineralogy and organic matter.
Iwasaki, Yuma*; Takeuchi, Ichiro*; Stanev, V.*; Gilad Kusne, A.*; Ishida, Masahiko*; Kirihara, Akihiro*; Ihara, Kazuki*; Sawada, Ryoto*; Terashima, Koichi*; Someya, Hiroko*; et al.
Scientific Reports (Internet), 9, p.2751_1 - 2751_7, 2019/02
Times Cited Count:61 Percentile:92.99(Multidisciplinary Sciences)Terashima, Motoki; Nagao, Seiya*; Iwatsuki, Teruki; Fujitake, Nobuhide*; Seida, Yoshimi*; Iijima, Kazuki; Yoshikawa, Hideki
Journal of Nuclear Science and Technology, 49(8), p.804 - 815, 2012/08
Times Cited Count:12 Percentile:66.54(Nuclear Science & Technology)Seida, Yoshimi; Terashima, Motoki; Tachi, Yukio; Iijima, Kazuki; Nakazawa, Toshiyuki*; Yamada, Norikazu*; Yui, Mikazu
Radiochimica Acta, 98(9-11), p.703 - 709, 2010/11
Times Cited Count:3 Percentile:24.08(Chemistry, Inorganic & Nuclear)Diffusion and sorption behaviors of Eu in sedimentary rock were investigated in the presence of humic substance in the present study. Sedimentary rock obtained from Horonobe URL test site in Hokkaido, Japan, (accessible pore diameter is around several to several ten nanometer) was used. The Diffusion behaviors of Eu were examined based on reservoir depletion method coupled with analysis of inner distribution of the radioactive elements in the rock. Time sequence of concentration of each radioactive element as well as the humic substance in the reservoir was obtained as a function of concentration and molecular size of humic substance. Coexistence of humic substance reduced the depletion of Eu in the reservoir, indicating complexation between the radioactive elements and the humic substance. On the contrary, obvious decrease of humic substance in the reservoir was not observed in the system. This observation suggests that the radioactive elements became hard to diffuse into the sedimentary rock due to an increase of their size through complexation with the humic substance. The sorption of Eu was reduced with increase of the humic substance although the sorption of the humic substance was not influenced by the existence of Th or Eu. The diffusion and sorption of Eu were found to be reduced in the presence of humic substance.
Terashima, Motoki; Seida, Yoshimi; Iwatsuki, Teruki; Iijima, Kazuki; Yoshikawa, Hideki; Yui, Mikazu; Nagao, Seiya*
no journal, ,
Eu(III) binding abilities of dissolved humic substances (HSs), which were extracted from deep groundwater in the Horonobe Underground Research Center, Hokkaido, Japan, were investigated by fluorescence quenching technique. In the synthetic groundwater condition, the fluorescence quenching due to the binding with Eu(III) was not observed for the groundwater HSs, while that was observed for the Aldrich humic acid (HA). This result indicates that the binding ability of the groundwater HSs are different from that of Aldrich HA. Based on structural characteristics of the groundwater HSs, the differences in the binding ability are discussed.
Murakami, Masao*; Demizu, Yusuke*; Niwa, Yasue*; Nagayama, Shinichi*; Maeda, Takuya*; Baba, Masashi*; Miyawaki, Daisuke*; Terashima, Kazuki*; Arimura, Takeshi*; Mima, Masayuki*; et al.
no journal, ,
Terashima, Motoki; Seida, Yoshimi*; Okazaki, Mitsuhiro; Iwatsuki, Teruki; Iijima, Kazuki; Yui, Mikazu
no journal, ,
no abstracts in English
Terashima, Motoki; Okazaki, Mitsuhiro; Iijima, Kazuki; Yui, Mikazu
no journal, ,
no abstracts in English
Terashima, Motoki; Okazaki, Mitsuhiro; Iijima, Kazuki; Yoshikawa, Hideki
no journal, ,
no abstracts in English
Terashima, Motoki; Saito, Takumi*; Iijima, Kazuki; Yui, Mikazu
no journal, ,
Terashima, Motoki; Tachi, Yukio; Saito, Takumi*; Iijima, Kazuki; Shimoda, Satoko*; Nakazawa, Toshiyuki*; Yoshikawa, Hideki
no journal, ,
no abstracts in English
Terashima, Motoki; Saito, Takumi*; Iijima, Kazuki; Yoshikawa, Hideki
no journal, ,
no abstracts in English
Terashima, Motoki; Saito, Takumi*; Okazaki, Mitsuhiro*; Tachi, Yukio; Iijima, Kazuki
no journal, ,
no abstracts in English
Terashima, Motoki; Tachi, Yukio; Fujiwara, Kenso; Iijima, Kazuki; Shimoda, Satoko*; Akagi, Yosuke*; Kato, Hiroyasu*
no journal, ,
no abstracts in English
Fujiwara, Kenso; Iijima, Kazuki; Terashima, Motoki; Tachi, Yukio
no journal, ,
no abstracts in English
Terashima, Motoki; Tachi, Yukio; Sato, Tomofumi; Akagi, Yosuke*; Kawamura, Makoto*; Nakane, Hideji*; Fujiwara, Kenso; Iijima, Kazuki
no journal, ,
no abstracts in English
Tachi, Yukio; Sato, Tomofumi; Terashima, Motoki; Takeda, Chizuko*; Fujiwara, Kenso; Iijima, Kazuki
no journal, ,
To predict the transport behaviors of radiocesium in river systems around the Fukushima Dai-ichi Nuclear Power Plant, the sorption and desorption of Cs on various types of river soils was investigated as functions of Cs concentration and reaction time. The key factors controlling the Cs transport were discussed based on types of clay minerals, organics, sorption kinetics, etc.
Fujiwara, Kenso; Iijima, Kazuki; Tachi, Yukio; Terashima, Motoki
no journal, ,
In the estuary, clay and/or soil particle with Cs is contacted by brackish water, and it is thought that Cs is desorbed from soil particle and clay particles are aggregated. In the case of last examination [1], I examined desorption and aggregation by using river soil and seawater, and measured the equilibrium data. In this study, kinetics data were measured.
Fujiwara, Kenso; Iijima, Kazuki; Terashima, Motoki; Tachi, Yukio
no journal, ,
Due to the accident of the Fukushima Dai-ichi Nuclear Power Plant, radioactive cesium (Cs) was released in a wide range of areas in and around the Fukushima prefecture. Thus, the chemical condition and mechanism governing transport behavior of Cs should be investigated to predict the behavior of Cs in the natural system. In the brackish water region near the river mouth where a cognate ion (Na, K) concentration increases due to the mixture of seawater, two dynamic evolutions influencing Cs behavior are expected. Firstly, the soil has an ion exchange sites, Cs adsorbed onto ion exchange sites of soil particles is likely to be desorbed. Secondly, decreasing of electrostatic repulsion among clay particles causes aggregation of particles leading to deposition with Cs. In this report, Cs desorption and aggregation of particles such as clays was examined using the seawater of the estuary in the coast and the sediments of the riverbed.