Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kobayashi, Taishi*; Teshima, Takeshi*; Sasaki, Takayuki*; Kitamura, Akira
Journal of Nuclear Science and Technology, 54(2), p.233 - 241, 2017/02
Times Cited Count:6 Percentile:51.46(Nuclear Science & Technology)Zr solubility in the presence of gluconic acid (GLU) and isosaccharinic acid (ISA) was investigated as a function of hydrogen ion concentration (pH ) and the total concentration of GLU or ISA. The dependence of the increase in Zr solubility on the pH
) and the total concentration of GLU or ISA. The dependence of the increase in Zr solubility on the pH and GLU concentration suggested the existence of Zr(OH)
and GLU concentration suggested the existence of Zr(OH) (GLU)
(GLU)
 in the neutral pH region and Zr(OH)
in the neutral pH region and Zr(OH) (GLU)(GLU
(GLU)(GLU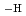 )
) in the alkaline pH region above pH
in the alkaline pH region above pH 10 as the dominant species in the presence of 10
10 as the dominant species in the presence of 10 - 10
- 10 mol/dm
mol/dm (M) GLU. In the presence of ISA, the dominant species Zr(OH)
(M) GLU. In the presence of ISA, the dominant species Zr(OH) (ISA)
(ISA)
 and Zr(OH)
and Zr(OH) (ISA)(ISA
(ISA)(ISA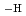 )
) were proposed to occur in the neutral and alkaline pH regions, similar to those found in the presence of GLU. From X-ray diffraction analysis, the solubility-limiting solid phase in the presence of GLU and ISA was considered to be Zr(OH)
were proposed to occur in the neutral and alkaline pH regions, similar to those found in the presence of GLU. From X-ray diffraction analysis, the solubility-limiting solid phase in the presence of GLU and ISA was considered to be Zr(OH) (am). The formation constants of the Zr gluconate and isosaccharinate complexes were determined by least squares fitting analysis of the solubility data, and the obtained values were discussed in comparison with those of tetravalent actinides.
(am). The formation constants of the Zr gluconate and isosaccharinate complexes were determined by least squares fitting analysis of the solubility data, and the obtained values were discussed in comparison with those of tetravalent actinides.
Yamamoto, Kazami; Hayashi, Naoki; Hiroki, Seiji; Saeki, Riuji; Toyama, Takeshi*; Teshima, Masaki*; Sato, Kenichiro*
Proceedings of 11th European Particle Accelerator Conference (EPAC '08) (CD-ROM), p.382 - 384, 2008/06
Nishikino, Masaharu; Ochi, Yoshihiro; Hasegawa, Noboru; Kawachi, Tetsuya; Ishino, Masahiko; Imazono, Takashi; Tanaka, Momoko; Sato, Katsutoshi; Yamamoto, Minoru; Oba, Toshiyuki; et al.
no journal, ,
Recent Progress in the development of laser-driven plasma soft X-ray laser (XRL) and the applications are presented. In the source development, TOPAZ laser using Nd:glass zigzag slab amplifiers with 10 J, 0.1 Hz repetition-rate routinely provides the fully spatial coherent X-ray laser at 13.9 nm for the applications. The bright, coherent, and picoseconds X-ray pulse can be used in a variety of applications such as X-ray speckle measurement and interferometer for probing materials, diffraction imaging, nano-scale fabrication, radiation biology, and so on. In order to extend the use of XRLs, now we are constructing new XRL beam lines for the optical pump and XRL probe experiment.
Kobayashi, Taishi*; Teshima, Takeshi*; Wang, P.*; Sasaki, Takayuki*; Kitamura, Akira
no journal, ,
We focused on the complexation of U(IV) and U(VI) with isosaccharinic acid (ISA) and measured U(IV) and U(VI) solubility in the presence of ISA. The solubility dependences on hydrogen ion concentration (pHc) and ISA concentration revealed the dominant U(IV) and U(VI) ISA complexes and determined their formation constants. Furthermore, the solubility of Zr(IV), as an analogous of An(IV), in the presence of organic acids with different number of hydroxyl groups were investigated to elucidate the strong complexation ability of ISA.