Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Zheng, X.*; Kato, Masaru*; Uemura, Yohei*; Matsumura, Daiju; Yagi, Ichizo*; Takahashi, Kiyonori*; Noro, Shinichiro*; Nakamura, Takayoshi*
Inorganic Chemistry, 62(3), p.1257 - 1263, 2023/01
Times Cited Count:1 Percentile:58.61(Chemistry, Inorganic & Nuclear)Yasuda, Satoshi; Tamura, Kazuhisa; Kato, Masaru*; Asaoka, Hidehito; Yagi, Ichizo*
Journal of Physical Chemistry C, 125(40), p.22154 - 22162, 2021/10
Times Cited Count:10 Percentile:57.69(Chemistry, Physical)Understanding electrochemical behavior of the alkaline metal cation-graphene interface in electrolyte is essential for understanding the fundamental electrochemical interface and development of graphene-based technologies. We report comprehensive analysis of the electrochemical behavior of both alkaline metal cations and graphene using electrochemical surface X-ray diffraction (EC-SXRD) and Raman (EC-Raman) spectroscopic techniques in which the interfacial structure of cations and the charging state and mechanical strain of the graphene can be elucidated. EC-SXRD and cyclic voltammetry demonstrated electrochemically driven specific adsorption and desorption of cations on the graphene surface involved in the dehydration and hydration process. This study provides new insight for understanding fundamental electrochemical behavior of the alkaline metal cation-graphene interface and contributes to the development of carbon-based novel applications.
Kato, Masaru*; Fujibayashi, Natsuki*; Abe, Daiki*; Matsubara, Naohiro*; Yasuda, Satoshi; Yagi, Ichizo*
ACS Catalysis, 11(4), p.2356 - 2365, 2021/02
Times Cited Count:36 Percentile:88.11(Chemistry, Physical)Fe-N-C oxygen reduction reaction catalyst is a key materials in polymer electrolyte fuel cell. However, the many Fe-N-C electrocatalysts still suffer from product selectivity due to the production of H O
O as the byproduct. In this work, we synthesized an ORR electrocatalyst of Cu
as the byproduct. In this work, we synthesized an ORR electrocatalyst of Cu , Fe
, Fe , and N-doped carbon nanotubes. This heterobimetallic catalyst showed the selective four electron reduction of O
, and N-doped carbon nanotubes. This heterobimetallic catalyst showed the selective four electron reduction of O to H
to H O. Kinetic analysis of the electrocatalytic ORR and hydrogen peroxide reduction reaction in acidic media revealed that Cu, Fe-N-doped catalyst showed two orders of magnitude higher rate constants for the direct four electron reduction of O
O. Kinetic analysis of the electrocatalytic ORR and hydrogen peroxide reduction reaction in acidic media revealed that Cu, Fe-N-doped catalyst showed two orders of magnitude higher rate constants for the direct four electron reduction of O to H
to H O than those for the two electron reduction of O
O than those for the two electron reduction of O to H
to H O
O , whereas a monometallic Fe-N-doped catalyst showed the same order of magnitude, indicating that the heterometallic cooperativity had a drastic impact on the ORR kinetics.
, whereas a monometallic Fe-N-doped catalyst showed the same order of magnitude, indicating that the heterometallic cooperativity had a drastic impact on the ORR kinetics.
Kato, Masaru*; Nakahoshiba, Ryota*; Ogura, Kazuya*; Tokuda, Shoichi*; Yasuda, Satoshi; Higashi, Kotaro*; Uruga, Tomoya*; Uemura, Yohei*; Yagi, Ichizo*
ACS Applied Energy Materials (Internet), 3(7), p.6768 - 6774, 2020/07
Times Cited Count:15 Percentile:62.59(Chemistry, Physical)To understand electronic effects of nitrogen-doped and polymer-coated carbon supports on the catalytic activity of Pt-based nanostructured catalysts, we prepared Pt Ni nanoframes (NFs) supported on polybenzimidazole (PBI)-coated and uncoated carbon nanotubes for the oxygen reduction reaction (ORR), and then compared their catalytic activities and electronic properties with those of NFs immobilized on nitrogen-doped and undoped carbon supports. Although both PBI-coating and nitrogen-doping approaches improved the catalytic activity of NFs,
Ni nanoframes (NFs) supported on polybenzimidazole (PBI)-coated and uncoated carbon nanotubes for the oxygen reduction reaction (ORR), and then compared their catalytic activities and electronic properties with those of NFs immobilized on nitrogen-doped and undoped carbon supports. Although both PBI-coating and nitrogen-doping approaches improved the catalytic activity of NFs, 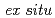 X-ray photoelectron spectroscopy and
X-ray photoelectron spectroscopy and  X-ray absorption spectroscopy revealed that nitrogen doping showed electronic effects on NFs, whereas PBI-coating showed almost no impact on the electronic state of NFs but stabilized Pt(OH)
X-ray absorption spectroscopy revealed that nitrogen doping showed electronic effects on NFs, whereas PBI-coating showed almost no impact on the electronic state of NFs but stabilized Pt(OH)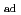 species under electrochemical conditions. Our studies demonstrate that difference in microscopic environments of nitrogen atoms at the catalyst/support interface is highly sensitive to the electronic effects of supports on Pt-based electrocatalysts.
species under electrochemical conditions. Our studies demonstrate that difference in microscopic environments of nitrogen atoms at the catalyst/support interface is highly sensitive to the electronic effects of supports on Pt-based electrocatalysts.
Yasuda, Satoshi; Tamura, Kazuhisa; Terasawa, Tomoo; Yano, Masahiro; Nakajima, Hideaki*; Morimoto, Takahiro*; Okazaki, Toshiya*; Agari, Ryushi*; Takahashi, Yasufumi*; Kato, Masaru*; et al.
Journal of Physical Chemistry C, 124(9), p.5300 - 5307, 2020/03
Times Cited Count:14 Percentile:60.14(Chemistry, Physical)Confinement of hydrogen molecules at graphene-substrate interface has presented significant importance from the viewpoints of development of fundamental understanding of two-dimensional material interface and energy storage system. In this study, we investigate H confinement at a graphene-Au interface by combining selective proton permeability of graphene and the electrochemical hydrogen evolution reaction (electrochemical HER) method. After HER on a graphene/Au electrode in protonic acidic solution, scanning tunneling microscopy finds that H
confinement at a graphene-Au interface by combining selective proton permeability of graphene and the electrochemical hydrogen evolution reaction (electrochemical HER) method. After HER on a graphene/Au electrode in protonic acidic solution, scanning tunneling microscopy finds that H nanobubble structures can be produced between graphene and the Au surface. Strain analysis by Raman spectroscopy also shows that atomic size roughness on the graphene/Au surface originating from the HER-induced strain relaxation of graphene plays significant role in formation of the nucleation site and H
nanobubble structures can be produced between graphene and the Au surface. Strain analysis by Raman spectroscopy also shows that atomic size roughness on the graphene/Au surface originating from the HER-induced strain relaxation of graphene plays significant role in formation of the nucleation site and H storage capacity.
storage capacity.
Kato, Masaru*; Muto, Marika*; Matsubara, Naohiro*; Uemura, Yohei*; Wakisaka, Yuki*; Yoneuchi, Tsubasa*; Matsumura, Daiju; Ishibara, Tomoko*; Tokushima, Takashi*; Noro, Shinichiro*; et al.
ACS Applied Energy Materials (Internet), 1(5), p.2358 - 2364, 2018/05
Times Cited Count:12 Percentile:43.4(Chemistry, Physical)Notsu, Hideo*; Umemura, Shun*; Tamura, Kazuhisa; Yagi, Ichizo*
no journal, ,
For more durable PEFC, the development of more active and durable catalyst must be required. For the purpose, the understanding of the interaction between catalyst surface and ionomer is needed. Cyclic voltammograms of Au single crystal electrode in electrolytes containing Nafion molecules are different from that of in sulfuric and perchloric acid solutions. It indicates that the phase transition process of the adsorbed Nafion molecules is different from that of sulfate and perchloride anions. However, the detail of surface process have not been understood. In this study, the adsorption process of Nafion molecule on a Au(111) electrode was monitored using SXS.
Yasuda, Satoshi; Terasawa, Tomoo; Yano, Masahiro; Ogawa, Hiroaki; Kato, Masaru*; Yagi, Ichizo*; Asaoka, Hidehito
no journal, ,
no abstracts in English