Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nakada, Akira; Kanai, Katsuta; Seya, Natsumi; Nishimura, Shusaku; Futagawa, Kazuo; Nemoto, Masashi; Tobita, Keiji; Yamada, Ryohei*; Uchiyama, Rei; Yamashita, Daichi; et al.
JAEA-Review 2022-078, 164 Pages, 2023/03
Environmental radiation monitoring around the Tokai Reprocessing Plant has been performed by the Nuclear Fuel Cycle Engineering Laboratories, based on "Safety Regulations for the Reprocessing Plant of Japan Atomic Energy Agency, Chapter IV - Environmental Monitoring". This annual report presents the results of the environmental monitoring and the dose estimation to the hypothetical inhabitant due to the radioactivity discharged from the plant to the atmosphere and the sea during April 2021 to March 2022. In this report, some data include the influence of the accidental release from the Fukushima Daiichi Nuclear Power Station of Tokyo Electric Power Co., Inc. (the trade name was changed to Tokyo Electric Power Company Holdings, Inc. on April 1, 2016) in March 2011. Appendices present comprehensive information, such as monitoring programs, monitoring methods, monitoring results and their trends, meteorological data and discharged radioactive wastes. In addition, the data which were influenced by the accidental release and exceeded the normal range of fluctuation in the monitoring, were evaluated.
Nakada, Akira; Nakano, Masanao; Kanai, Katsuta; Seya, Natsumi; Nishimura, Shusaku; Nemoto, Masashi; Tobita, Keiji; Futagawa, Kazuo; Yamada, Ryohei; Uchiyama, Rei; et al.
JAEA-Review 2021-062, 163 Pages, 2022/02
Environmental radiation monitoring around the Tokai Reprocessing Plant has been performed by the Nuclear Fuel Cycle Engineering Laboratories, based on "Safety Regulations for the Reprocessing Plant of Japan Atomic Energy Agency, Chapter IV - Environmental Monitoring". This annual report presents the results of the environmental monitoring and the dose estimation to the hypothetical inhabitant due to the radioactivity discharged from the plant to the atmosphere and the sea during April 2020 to March 2021. In this report, some data include the influence of the accidental release from the Fukushima Daiichi Nuclear Power Station of Tokyo Electric Power Co., Inc. (the trade name was changed to Tokyo Electric Power Company Holdings, Inc. on April 1, 2016) in March 2011. Appendices present comprehensive information, such as monitoring programs, monitoring methods, monitoring results and their trends, meteorological data and discharged radioactive wastes. In addition, the data which were influenced by the accidental release and exceeded the normal range of fluctuation in the monitoring, were evaluated.
Igarashi, Go*; Haga, Kazuko*; Yamada, Kazuo*; Aihara, Haruka; Shibata, Atsuhiro; Koma, Yoshikazu; Maruyama, Ippei*
Journal of Advanced Concrete Technology, 19(9), p.950 - 976, 2021/09
Times Cited Count:5 Percentile:39.74(Construction & Building Technology)Kitazato, Kohei*; Milliken, R. E.*; Iwata, Takahiro*; Abe, Masanao*; Otake, Makiko*; Matsuura, Shuji*; Takagi, Yasuhiko*; Nakamura, Tomoki*; Hiroi, Takahiro*; Matsuoka, Moe*; et al.
Nature Astronomy (Internet), 5(3), p.246 - 250, 2021/03
Times Cited Count:43 Percentile:96.93(Astronomy & Astrophysics)Here we report observations of Ryugu's subsurface material by the Near-Infrared Spectrometer (NIRS3) on the Hayabusa2 spacecraft. Reflectance spectra of excavated material exhibit a hydroxyl (OH) absorption feature that is slightly stronger and peak-shifted compared with that observed for the surface, indicating that space weathering and/or radiative heating have caused subtle spectral changes in the uppermost surface. However, the strength and shape of the OH feature still suggests that the subsurface material experienced heating above 300  C, similar to the surface. In contrast, thermophysical modeling indicates that radiative heating does not increase the temperature above 200
C, similar to the surface. In contrast, thermophysical modeling indicates that radiative heating does not increase the temperature above 200  C at the estimated excavation depth of 1 m, even if the semimajor axis is reduced to 0.344 au. This supports the hypothesis that primary thermal alteration occurred due to radiogenic and/or impact heating on Ryugu's parent body.
C at the estimated excavation depth of 1 m, even if the semimajor axis is reduced to 0.344 au. This supports the hypothesis that primary thermal alteration occurred due to radiogenic and/or impact heating on Ryugu's parent body.
Yamada, Kazuo*; Maruyama, Ippei*; Haga, Kazuko*; Igarashi, Go*; Aihara, Haruka; Tomita, Sayuri*; Kiran, R.*; Osawa, Norihisa*; Shibata, Atsuhiro; Shibuya, Kazutoshi*; et al.
Proceedings of International Waste Management Symposia 2021 (WM2021) (CD-ROM), 10 Pages, 2021/03
Kitazato, Kohei*; Milliken, R. E.*; Iwata, Takahiro*; Abe, Masanao*; Otake, Makiko*; Matsuura, Shuji*; Arai, Takehiko*; Nakauchi, Yusuke*; Nakamura, Tomoki*; Matsuoka, Moe*; et al.
Science, 364(6437), p.272 - 275, 2019/04
Times Cited Count:259 Percentile:99.73(Multidisciplinary Sciences)The near-Earth asteroid 162173 Ryugu, the target of Hayabusa2 sample return mission, is believed to be a primitive carbonaceous object. The Near Infrared Spectrometer (NIRS3) on Hayabusa2 acquired reflectance spectra of Ryugu's surface to provide direct measurements of the surface composition and geological context for the returned samples. A weak, narrow absorption feature centered at 2.72 micron was detected across the entire observed surface, indicating that hydroxyl (OH)-bearing minerals are ubiquitous there. The intensity of the OH feature and low albedo are similar to thermally- and/or shock-metamorphosed carbonaceous chondrite meteorites. There are few variations in the OH-band position, consistent with Ryugu being a compositionally homogeneous rubble-pile object generated from impact fragments of an undifferentiated aqueously altered parent body.
Yamada, Kazuo*; Maruyama, Ippei*; Koma, Yoshikazu; Haga, Kazuko*; Igarashi, Go*; Shibuya, Kazutoshi*; Aihara, Haruka
Proceedings of International Waste Management Symposia 2019 (WM2019) (CD-ROM), 6 Pages, 2019/03
Sakamoto, Hiroyuki*; Akagi, Yosuke*; Yamada, Kazuo*; Tachi, Yukio; Fukuda, Daisuke*; Ishimatsu, Koichi*; Matsuda, Mikiya*; Saito, Nozomi*; Uemura, Jitsuya*; Namihira, Takao*; et al.
Nihon Genshiryoku Gakkai Wabun Rombunshi, 17(2), p.57 - 66, 2018/05
Concrete debris contaminated with radioactive cesium and other nuclides have been generated from the accident in the Fukushima Dai-ichi Nuclear Power Plant and there will be generated due to the decommissioning of nuclear power plants in the future. Although conventional decontamination techniques are effective for flat concrete surfaces such as floors and walls, it is not clear what techniques to apply for decontaminating radioactive concrete debris. In this study, focusing on a pulsed power discharge technique, fundamental experimental works were carried out. Decontamination of concrete by applying the aggregate recycling technique using the pulsed power discharge technique was evaluated by measuring radioactivity of aggregate and sludge separated from the contaminated concrete. The results suggest that the separation into aggregate and sludge of the contaminated concrete debris could achieve decontamination and volume reduction of the radioactive concrete debris.
 V
V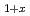 Al alloys
Al alloysSoda, Kazuo*; Harada, Shota*; Hayashi, Toshimitsu*; Kato, Masahiko*; Ishikawa, Fumihiro*; Yamada, Yu*; Fujimori, Shinichi; Saito, Yuji
Materials Transactions, 57(7), p.1040 - 1044, 2016/06
Times Cited Count:2 Percentile:12.27(Materials Science, Multidisciplinary)Kusaka, Katsuhiro*; Hosoya, Takaaki*; Yamada, Taro*; Tomoyori, Katsuaki; Ohara, Takashi; Katagiri, Masaki*; Kurihara, Kazuo; Tanaka, Ichiro*; Niimura, Nobuo*
Journal of Synchrotron Radiation, 20(6), p.994 - 998, 2013/11
Times Cited Count:37 Percentile:85.99(Instruments & Instrumentation)The IBARAKI biological crystal diffractometer, iBIX, is a high-performance time-of-flight neutron single-crystal diffractometer for elucidating mainly the hydrogen, protonation and hydration structures of biological macromolecules in various life processes. Since the end of 2008, iBIX has been available to user's experiments supported by Ibaraki University. Since August 2012, an upgrade of the 14-existing detectors has begun and 16 new detectors have been installed for iBIX. The total measurement efficiency of the present diffractometer has been impoved by one order of magnitude from the previous one with the increasing of accelerator power. In December 2012, commissioning of the new detectors was successful, and collection of the diffraction dataset of ribonucrease A as a standard protein was attempted in order to estimate the performance of the upgraded iBIX in comparison with previous results. The resolution of diffraction data, equivalence among intensities of symmetry-related reflections and reliability of the refined structure have been improved dramatically. iBIX is expected to be one of the highest-performance neutron single-crystal diffractometers for biological macromolecules in the world.
Yokoyama, Takeshi*; Mizuguchi, Mineyuki*; Nabeshima, Yuko*; Kusaka, Katsuhiro*; Yamada, Taro*; Hosoya, Takaaki*; Ohara, Takashi; Kurihara, Kazuo; Tanaka, Ichiro*; Niimura, Nobuo*
Journal of Synchrotron Radiation, 20(6), p.834 - 837, 2013/11
Times Cited Count:6 Percentile:33.62(Instruments & Instrumentation)Transthyretin (TTR) is a tetrameric protein. TTR misfolding and aggregation are associated with human amyloid diseases. Dissociation of the TTR tetramer is believed to be the rate-limiting step in the amyloid fibril formation cascade. Low pH is known to promote dissociation into monomer and the formation of amyloid fibrils. In order to reveal the molecular mechanisms underlying pH sensitivity and structural stabilities of TTR, neutron diffraction studies were conducted using the IBARAKI Biological Crystal Diffractometer with the time-of-flight method. Crystals for the neutron diffraction experiments were grown up to 2.5 mm for four months. The neutron crystal structure solved at 2.0
for four months. The neutron crystal structure solved at 2.0  revealed the protonation states of His88 and the detailed hydrogen-bond network depending on the protonation states of His88. This hydrogen-bond network is involved in monomer-monomer and dimer-dimer interactions, suggesting that the double protonation of His88 by acidification breaks the hydrogen-bond network and causes the destabilization of the TTR tetramer. Structural comparison with the X-ray crystal structure at acidic pH identified the three amino acid residues responsible for the pH sensitivity of TTR. Our neutron model provides insights into the molecular stability related to amyloidosis.
revealed the protonation states of His88 and the detailed hydrogen-bond network depending on the protonation states of His88. This hydrogen-bond network is involved in monomer-monomer and dimer-dimer interactions, suggesting that the double protonation of His88 by acidification breaks the hydrogen-bond network and causes the destabilization of the TTR tetramer. Structural comparison with the X-ray crystal structure at acidic pH identified the three amino acid residues responsible for the pH sensitivity of TTR. Our neutron model provides insights into the molecular stability related to amyloidosis.
Kondo, Hiroaki*; Yamada, Tetsuji*; Chino, Masamichi; Iwasaki, Toshiki*; Katata, Genki; Maki, Takashi*; Saito, Kazuo*; Terada, Hiroaki; Tsuruta, Haruo*
Tenki, 60(9), p.723 - 729, 2013/09
no abstracts in English
 -thrombin-bivalirubin complex at pD 5.0; Protonation states and hydration structure of the enzyme-product complex
-thrombin-bivalirubin complex at pD 5.0; Protonation states and hydration structure of the enzyme-product complexYamada, Taro*; Kurihara, Kazuo; Onishi, Yuki*; Tamada, Taro; Tomoyori, Katsuaki; Masumi, Kenji*; Tanaka, Ichiro*; Kuroki, Ryota; Niimura, Nobuo*
Biochimica et Biophysica Acta; Proteins and Proteomics, 1834(8), p.1532 - 1538, 2013/08
Times Cited Count:17 Percentile:48.69(Biochemistry & Molecular Biology)The protonation states and hydration structures of the  -thrombin-bivalirubin complex were studied by joint XN refinement of the single crystal X-ray and neutron diffraction data at resolutions of 1.6 and 2.8
-thrombin-bivalirubin complex were studied by joint XN refinement of the single crystal X-ray and neutron diffraction data at resolutions of 1.6 and 2.8  , respectively. The atomic distances were estimated by carrying out X-ray crystallographic analysis at 1.25
, respectively. The atomic distances were estimated by carrying out X-ray crystallographic analysis at 1.25  resolution. The complex represents a model of the enzyme-product (EP) complex of
resolution. The complex represents a model of the enzyme-product (EP) complex of  -thrombin. The neutron scattering length maps around the active site suggest that the side chain of H57/H was deuterated. The joint XN refinement showed that occupancies for D
-thrombin. The neutron scattering length maps around the active site suggest that the side chain of H57/H was deuterated. The joint XN refinement showed that occupancies for D 1 and D
1 and D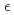 2 of H57/H were 1.0 and 0.7, respectively. However, no significant neutron scattering length density was observed around the hydroxyl oxygen O
2 of H57/H were 1.0 and 0.7, respectively. However, no significant neutron scattering length density was observed around the hydroxyl oxygen O of S195/H, which was close to the carboxylic carbon atom of dFPR-COOH. These observations suggest that the O
of S195/H, which was close to the carboxylic carbon atom of dFPR-COOH. These observations suggest that the O atom of S195/H is deprotonated and maintains its nucleophilicity in the EP complex. In addition to the active site, the hydration structures of the S1 subsite and the Exosite I, which are involved in the recognition of bivalirudin, are presented.
atom of S195/H is deprotonated and maintains its nucleophilicity in the EP complex. In addition to the active site, the hydration structures of the S1 subsite and the Exosite I, which are involved in the recognition of bivalirudin, are presented.
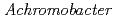 protease I at pD 8.0; Protonation states and hydration structure in the free-form
protease I at pD 8.0; Protonation states and hydration structure in the free-formOnishi, Yuki*; Yamada, Taro*; Kurihara, Kazuo; Tanaka, Ichiro*; Sakiyama, Fumio*; Masaki, Takeharu*; Niimura, Nobuo*
Biochimica et Biophysica Acta; Proteins and Proteomics, 1834(8), p.1642 - 1647, 2013/08
Times Cited Count:8 Percentile:20.86(Biochemistry & Molecular Biology)The structure of the free-form of 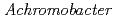 protease I (API) at pD 8.0 was refined by simultaneous use of single crystal X-ray and neutron diffraction data sets to investigate the protonation states of key catalytic residues of the serine protease. Occupancy refinement of the catalytic triad in the active site of API free-form showed that ca. 30% of the imidazole ring of H57 and ca. 70% of the hydroxyl group of S194 were deuterated. This observation indicates that a major fraction of S194 is protonated in the absence of a substrate. The protonation state of the catalytic triad in API was compared with the bovine
protease I (API) at pD 8.0 was refined by simultaneous use of single crystal X-ray and neutron diffraction data sets to investigate the protonation states of key catalytic residues of the serine protease. Occupancy refinement of the catalytic triad in the active site of API free-form showed that ca. 30% of the imidazole ring of H57 and ca. 70% of the hydroxyl group of S194 were deuterated. This observation indicates that a major fraction of S194 is protonated in the absence of a substrate. The protonation state of the catalytic triad in API was compared with the bovine  -trypsin-BPTI complex. The comparison led to the hypothesis that close contact of a substrate with S194 could lower the acidity of its hydroxyl group, thereby allowing H57 to extract the hydrogen from the hydroxyl group of S194. H210, which is a residue specific to API, does not form a hydrogen bond with the catalytic triad residue D113. Instead, H210 forms a hydrogen bond network with S176, H177 and a water molecule. The close proximity of the bulky, hydrophobic residue W169 may protect this hydrogen bond network, and this protection may stabilize the function of API over a wide pH range.
-trypsin-BPTI complex. The comparison led to the hypothesis that close contact of a substrate with S194 could lower the acidity of its hydroxyl group, thereby allowing H57 to extract the hydrogen from the hydroxyl group of S194. H210, which is a residue specific to API, does not form a hydrogen bond with the catalytic triad residue D113. Instead, H210 forms a hydrogen bond network with S176, H177 and a water molecule. The close proximity of the bulky, hydrophobic residue W169 may protect this hydrogen bond network, and this protection may stabilize the function of API over a wide pH range.
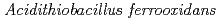
Kanao, Tadayoshi*; Kosaka, Megumi*; Yoshida, Kyoya*; Nakayama, Hisayuki*; Tamada, Taro; Kuroki, Ryota; Yamada, Hidenori; Takada, Jun*; Kamimura, Kazuo*
Acta Crystallographica Section F, 69(6), p.692 - 694, 2013/06
Times Cited Count:10 Percentile:59.68(Biochemical Research Methods)Tetrathionate hydrolase (4THase) from the iron- and sulfur-oxidizing bacterium 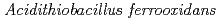 catalyses the disproportionate hydrolysis of tetrathionate to elemental sulfur, thiosulfate and sulfate. The gene encoding 4THase (
catalyses the disproportionate hydrolysis of tetrathionate to elemental sulfur, thiosulfate and sulfate. The gene encoding 4THase (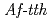 ) was expressed as inclusion bodies in recombinant
) was expressed as inclusion bodies in recombinant 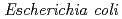 . Recombinant
. Recombinant 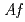 -Tth was activated by refolding under acidic conditions and was then purified to homogeneity. The recombinant protein was crystallized in 20 m
-Tth was activated by refolding under acidic conditions and was then purified to homogeneity. The recombinant protein was crystallized in 20 m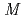 glycine buffer pH 10 containing 50 m
glycine buffer pH 10 containing 50 m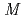 sodium chloride and 33%(
sodium chloride and 33%(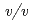 ) PEG 1000 using the hanging-drop vapour-diffusion method. The crystal was a hexagonal cylinder with dimensions of 0.2
) PEG 1000 using the hanging-drop vapour-diffusion method. The crystal was a hexagonal cylinder with dimensions of 0.2  0.05
0.05  0.05 mm. X-ray crystallographic analysis showed that the crystal diffracted to 2.15
0.05 mm. X-ray crystallographic analysis showed that the crystal diffracted to 2.15  resolution and belongs to space group
resolution and belongs to space group 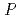 3
3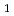 or
or 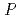 3
3 , with unit-cell parameters
, with unit-cell parameters 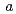 =
= 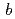 = 92.1,
= 92.1, 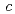 = 232.6
= 232.6  .
.
 H
H ONH
ONH PbBr
PbBr studied using single-crystal neutron diffraction
studied using single-crystal neutron diffractionKawasaki, Takuro; Takahashi, Miwako*; Ohara, Takashi*; Tanaka, Ichiro*; Kusaka, Katsuhiro*; Hosoya, Takaaki*; Yamada, Taro*; Kurihara, Kazuo
Journal of the Physical Society of Japan, 81(9), p.094602_1 - 094602_6, 2012/09
Times Cited Count:7 Percentile:46.7(Physics, Multidisciplinary)Yokoyama, Takeshi*; Mizuguchi, Mineyuki*; Nabeshima, Yuko*; Kusaka, Katsuhiro*; Yamada, Taro*; Hosoya, Takaaki*; Ohara, Takashi*; Kurihara, Kazuo; Tomoyori, Katsuaki*; Tanaka, Ichiro*; et al.
Journal of Structural Biology, 177(2), p.283 - 290, 2012/02
Times Cited Count:48 Percentile:83.41(Biochemistry & Molecular Biology)Kawamura, Kenji*; Yamada, Taro*; Kurihara, Kazuo; Tamada, Taro; Kuroki, Ryota; Tanaka, Ichiro*; Takahashi, Haruyuki*; Niimura, Nobuo*
Acta Crystallographica Section D, 67(2), p.140 - 148, 2011/02
Times Cited Count:28 Percentile:88.53(Biochemical Research Methods)Tanaka, Ichiro*; Kusaka, Katsuhiro*; Hosoya, Takaaki*; Niimura, Nobuo*; Ohara, Takashi*; Kurihara, Kazuo; Yamada, Taro*; Onishi, Yuki*; Tomoyori, Katsuaki*; Yokoyama, Takeshi*
Acta Crystallographica Section D, 66(11), p.1194 - 1197, 2010/11
Times Cited Count:49 Percentile:94.6(Biochemical Research Methods)The IBARAKI Biological Crystal Diffractometer (iBIX), a new diffractometer for protein crystallography at the next-generation neutron source at J-PARC (Japan Proton Accelerator Research Complex), has been constructed and has been operational since December 2008. Preliminary structure analyses of organic crystals showed that iBIX has high performance even at 120 kW operation and the first full data set is being collected from a protein crystal.
 resolution
resolutionYagi, Daichi*; Yamada, Taro*; Kurihara, Kazuo; Onishi, Yuki*; Yamashita, Masahiro*; Tamada, Taro; Tanaka, Ichiro*; Kuroki, Ryota; Niimura, Nobuo*
Acta Crystallographica Section D, 65(9), p.892 - 899, 2009/09
Times Cited Count:17 Percentile:80.39(Biochemical Research Methods)A neutron crystallographic analysis of phosphate-free bovine pancreatic RNase A has been carried out at 1.7  resolution using the BIX-4 single-crystal diffractometer at the JRR-3 reactor of the Japan Atomic Energy Agency. The high resolution structural model allowed us to determine that His12 acts mainly as a general base in the catalytic process of RNase A. Numerous other distinctive structural features such as the hydrogen positions of methyl groups, hydroxyl groups, prolines, asparagines and glutamines were also determined at 1.7
resolution using the BIX-4 single-crystal diffractometer at the JRR-3 reactor of the Japan Atomic Energy Agency. The high resolution structural model allowed us to determine that His12 acts mainly as a general base in the catalytic process of RNase A. Numerous other distinctive structural features such as the hydrogen positions of methyl groups, hydroxyl groups, prolines, asparagines and glutamines were also determined at 1.7  resolution. The protonation and deprotonation states of all of the charged amino-acid residues allowed us to provide a definitive description of the hydrogen-bonding network around the active site and the H atoms of the key His48 residue. Differences in hydrogen-bond strengths for the
resolution. The protonation and deprotonation states of all of the charged amino-acid residues allowed us to provide a definitive description of the hydrogen-bonding network around the active site and the H atoms of the key His48 residue. Differences in hydrogen-bond strengths for the  -helices and
-helices and  -sheets were inferred from determination of the hydrogen-bond lengths and the H/D-exchange ratios of the backbone amide H atoms. The correlation between the B factors and hydrogen-bond lengths of the hydration water molecules was also determined.
-sheets were inferred from determination of the hydrogen-bond lengths and the H/D-exchange ratios of the backbone amide H atoms. The correlation between the B factors and hydrogen-bond lengths of the hydration water molecules was also determined.