Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 species at SiO
species at SiO /Si interfaces in Si dry oxidation; Comparison between p-Si(001) and n-Si(001) surfaces
/Si interfaces in Si dry oxidation; Comparison between p-Si(001) and n-Si(001) surfacesTsuda, Yasutaka; Yoshigoe, Akitaka; Ogawa, Shuichi*; Sakamoto, Tetsuya*; Yamamoto, Yoshiki*; Yamamoto, Yukio*; Takakuwa, Yuji*
Journal of Chemical Physics, 157(23), p.234705_1 - 234705_21, 2022/12
Times Cited Count:0 Percentile:0.01(Chemistry, Physical)Yamamoto, Masahiro; Soma, Yasutaka; Igarashi, Takahiro; Ueno, Fumiyoshi
Proceedings of Annual Congress of the European Federation of Corrosion (EUROCORR 2018) (USB Flash Drive), 7 Pages, 2018/09
In order to clarify the SCC behavior of SUS316L under BWR environment, mass transfer inside crevice of SUS316L in high temperature water using various crevice gap samples was investigated. The samples were prepared by put together two SUS316L sheets. Crevice gap differs from 0.005 mm to 0.1 mm. Corrosion tests were conducted in 8 ppm dissolved oxygen (DO) conditions. Surface oxide film was analysed by laser Raman spectroscopy (LRS) after immersion. Numerical simulations were also conducted by using COMSOL Maltiphysics. Diffusion process of DO and the other chemical species were calculated with connected to electrochemical process. Electrical conductivities inside the crevice were 100 times larger than these of outer water. The reason of high conductivity is existence of Fe ions at the DO depletion crevice.
ions at the DO depletion crevice.
Yamamoto, Masahiro; Sato, Tomonori; Igarashi, Takahiro; Ueno, Fumiyoshi; Soma, Yasutaka
Proceedings of European Corrosion Congress 2017 (EUROCORR 2017) and 20th ICC & Process Safety Congress 2017 (USB Flash Drive), 6 Pages, 2018/09
The authors have studied the differences between outer surface and the crevice-like portion of SUS316L in high pressurized and high temperature water containing dissolved oxygen. We have already introduced that changes in the characteristics of corrosion products along the crevice directions and gap width. It is suggested that the environmental conditions are different with the features of crevice from these results. In this report, we introduce the changes in oxide films with crevice gaps and comparison with the numerical simulation data utilizing of FEM calculation.
Soma, Yasutaka; Kato, Chiaki; Yamamoto, Masahiro
Corrosion, 70(4), p.366 - 374, 2014/04
Times Cited Count:9 Percentile:42.07(Materials Science, Multidisciplinary)Surface oxide layers were formed within crevices of type 316L stainless steels in pure water at 288 C and 8 MPa. Cross-sectional structures of the surface oxides were analyzed using transmission electron microscopy. In the condition of dissolved oxygen concentration of 2 ppm, the properties of the surface oxide layer changed with position and dual or triplex layered oxides were formed at a certain distance from the crevice mouth. The multilayered oxides were composed of Fe-based oxide in the core and a high-Cr content in the outer layer, which had not been observed on a boldly exposed surface. On the contrary, in deaerated condition, the surface oxide layers were composed of a Fe
C and 8 MPa. Cross-sectional structures of the surface oxides were analyzed using transmission electron microscopy. In the condition of dissolved oxygen concentration of 2 ppm, the properties of the surface oxide layer changed with position and dual or triplex layered oxides were formed at a certain distance from the crevice mouth. The multilayered oxides were composed of Fe-based oxide in the core and a high-Cr content in the outer layer, which had not been observed on a boldly exposed surface. On the contrary, in deaerated condition, the surface oxide layers were composed of a Fe O
O -based outer and a Cr-enriched inner oxide layer, regardless of the crevice position. Electrochemical condition within the crevice was identified by using E-pH diagram. It was suggested that, at 400
-based outer and a Cr-enriched inner oxide layer, regardless of the crevice position. Electrochemical condition within the crevice was identified by using E-pH diagram. It was suggested that, at 400 m distance from the crevice mouth, the potential lowered at the early stage of exposure and then, shifted to noble direction with decrement of pH. Consequently, even within a narrow crevice with a gap size of a few
m distance from the crevice mouth, the potential lowered at the early stage of exposure and then, shifted to noble direction with decrement of pH. Consequently, even within a narrow crevice with a gap size of a few  m, the uniqueness of the crevice electrochemistry, characterized by the position and time dependence of both the potential and the pH, has been exhibited.
m, the uniqueness of the crevice electrochemistry, characterized by the position and time dependence of both the potential and the pH, has been exhibited.
Soma, Yasutaka; Kato, Chiaki; Yamamoto, Masahiro
Journal of the Electrochemical Society, 159(8), p.C334 - C340, 2012/07
Times Cited Count:9 Percentile:31.71(Electrochemistry)Surface oxide layers on stainless steel were formed in 561 K pure water at different potentials. To understand the oxide's properties in terms of their potential dependence, cross-sectional views of the oxide layer were analyzed using an electron microprobe technique and potential-solubility (equilibrium concentration of ionic species) diagram. In the potential range investigated, duplex oxide layers composed of mono- and bimetallic oxide were formed. Both the structure and composition of the oxide layer were affected by solubility of oxides.
 C water
C waterSoma, Yasutaka; Kato, Chiaki; Yamamoto, Masahiro
Materials Transactions, 53(1), p.195 - 200, 2012/01
Times Cited Count:9 Percentile:47.24(Materials Science, Multidisciplinary)Surface oxide layer on SUS316L stainless steels exposed to 288 C pure water with 2ppm dissolved oxygen (DO) for 1
C pure water with 2ppm dissolved oxygen (DO) for 1 100h were analyzed using Focused Ion Beam and Scanning Transmission Electron Microscope equipped with EDS to understand the early stage of surface oxide layer formation. At 1h exposure, double oxide layer which is composed of compact inner oxide layer and outer oxide layer with Fe-rich and Ni-rich oxide particles was formed. At the outermost region of the SUS316L substrate, Ni and Cr were enriched. At 100h exposure, growth of the inner oxide layer was suppressed and the Ni and Cr enriched region at the alloy substrate was preserved underneath the Ni-rich outer oxide particles. At 1h exposure, most of the outer oxide particles were composed of Fe-rich ones, at 10h exposure, another Ni-rich outer oxide particles were nucleated and grew faster than Fe-rich ones. Consequently, a part of pre-formed Fe-rich outer oxide particles were covered with Ni-rich ones.
100h were analyzed using Focused Ion Beam and Scanning Transmission Electron Microscope equipped with EDS to understand the early stage of surface oxide layer formation. At 1h exposure, double oxide layer which is composed of compact inner oxide layer and outer oxide layer with Fe-rich and Ni-rich oxide particles was formed. At the outermost region of the SUS316L substrate, Ni and Cr were enriched. At 100h exposure, growth of the inner oxide layer was suppressed and the Ni and Cr enriched region at the alloy substrate was preserved underneath the Ni-rich outer oxide particles. At 1h exposure, most of the outer oxide particles were composed of Fe-rich ones, at 10h exposure, another Ni-rich outer oxide particles were nucleated and grew faster than Fe-rich ones. Consequently, a part of pre-formed Fe-rich outer oxide particles were covered with Ni-rich ones.
 C pure water
C pure waterSoma, Yasutaka; Kato, Chiaki; Yamamoto, Masahiro
no journal, ,
Surface oxide layers on stainless steel were formed in 288 C pure water at different potentials. To understand the oxide's properties in terms of their potential dependence, cross-sectional views of the oxide layer were analyzed using an electron microprobe technique and potential-solubility (equilibrium concentration of ionic species) diagram. In the potential range investigated, duplex oxide layers composed of mono- and bimetallic oxide were formed. Both the structure and composition of the oxide layer were affected by solubility of oxides.
C pure water at different potentials. To understand the oxide's properties in terms of their potential dependence, cross-sectional views of the oxide layer were analyzed using an electron microprobe technique and potential-solubility (equilibrium concentration of ionic species) diagram. In the potential range investigated, duplex oxide layers composed of mono- and bimetallic oxide were formed. Both the structure and composition of the oxide layer were affected by solubility of oxides.
Soma, Yasutaka; Kato, Chiaki; Yamamoto, Masahiro
no journal, ,
Surface oxide layers on crevice of 316L stainless steels in high temperature and high pressure water containing 2ppm dissolved oxygen (DO) were investigated by using SEM, STEM, and Raman spectroscopy. Effects of distance from open-mouse of the crevices (Y) and corresponding DO concentration on surface oxide layer were studied. Depending on the Y value, four different regions with different surface oxide layer were observed if the crevice were sufficiently narrow. The oxide layers were consisted of inner and outer oxide layer at the all regions. Change in composition of outer oxide layer clearly showed that the DO concentration decreased with increasing Y value. However, thickness of the inner oxide layer showed maximum value at certain Y value and then, decreased with the Y value. This behavior considered to be brought by differences in the DO concentration, ionic concentration and pH.
Soma, Yasutaka; Kato, Chiaki; Yamamoto, Masahiro
no journal, ,
Surface oxide layers were formed within crevices of type 316L stainless steels in pure water at 561 K and 8 MPa. Cross-sectional structures of the surface oxides were analyzed using transmission electron microscopy. Under the condition of a dissolved oxygen concentration of 2 ppm, the properties of the surface oxide layer changed with the crevice position and dual or triplex layered oxides were formed, which had not been observed on a boldly exposed surface. The thickness of the inner oxide layer showed a local maximum at a certain distance from the crevice mouth. These oxide characteristics suggest the presence of locally varying electrochemical conditions. Potential-pH diagrams of the multilayered oxides suggest that the potential decreased at the early stage of exposure and then re-increased, accompanied by weak acidification.
Soma, Yasutaka; Ueno, Fumiyoshi; Yamamoto, Masahiro
no journal, ,
Diffusion behavior of dissolved oxygen into crevice of stainless steel in high temperature is very important to understand crevice environment. In this research, we developed 3D model of crevice and using it, we carried out numerical simulation of dissolved oxygen diffusion into the crevice. The result of numerical simulation showed good agreement with experimentally obtained result.
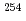 Es region by Coulomb excitation
Es region by Coulomb excitation  -ray spectroscopy
-ray spectroscopyYanagihara, Rikuto; Ideguchi, Eiji*; Nishio, Katsuhisa; Orlandi, R.; Makii, Hiroyuki; Asai, Masato; Hirose, Kentaro; Tsukada, Kazuaki; Toyoshima, Atsushi; Sato, Tetsuya; et al.
no journal, ,
 Cf by Coulomb excitation gamma-ray spectroscopy
Cf by Coulomb excitation gamma-ray spectroscopyPham, T. T.*; Yanagihara, Rikuto*; Ideguchi, Eiji*; Orlandi, R.; Nishio, Katsuhisa; Makii, Hiroyuki; Asai, Masato; Hirose, Kentaro; Tsukada, Kazuaki; Toyoshima, Atsushi*; et al.
no journal, ,
 /Si(001) interfacial oxidation
/Si(001) interfacial oxidationTsuda, Yasutaka; Ogawa, Shuichi*; Yoshigoe, Akitaka; Tominaga, Aki; Sakamoto, Tetsuya; Yamamoto, Yoshiki*; Yamamoto, Yukio*; Takakuwa, Yuji*
no journal, ,
no abstracts in English
 species at SiO
species at SiO /Si interfaces in Si dry oxidation
/Si interfaces in Si dry oxidationTsuda, Yasutaka; Yoshigoe, Akitaka; Ogawa, Shuichi*; Sakamoto, Tetsuya*; Yamamoto, Yoshiki*; Yamamoto, Yukio*; Takakuwa, Yuji*
no journal, ,
no abstracts in English