Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yamashita, Keishiro*; Nakayama, Kazuya*; Komatsu, Kazuki*; Ohara, Takashi; Munakata, Koji*; Hattori, Takanori; Sano, Asami; Kagi, Hiroyuki*
Acta Crystallographica Section B; Structural Science, Crystal Engineering and Materials (Internet), 79(5), p.414 - 426, 2023/10
Times Cited Count:0 Percentile:0.02(Chemistry, Multidisciplinary)The structure of a recently-found hyperhydrated form of sodium chloride, NaCl 13H(D)
13H(D) O, has been determined by
O, has been determined by  single-crystal neutron diffraction at 1.7 GPa and 298 K. It has large hydrogen-bond networks and some water molecules have distorted bonding features such as bifurcated hydrogen bonds and five-coordinated water molecules. The hydrogen-bond network has similarities to ice VI in terms of network topology and disordered hydrogen bonds. Assuming the equivalence of network components connected by pseudo symmetries, the overall network structure of this hydrate can be expressed by breaking it down into smaller structural units which correspond to the ice VI network structure. This hydrogen-bond network contains orientational disorder of water molecules in contrast to the known salt hydrates. Here, we present an example for further insights into a hydrogen-bond network containing ionic species.
single-crystal neutron diffraction at 1.7 GPa and 298 K. It has large hydrogen-bond networks and some water molecules have distorted bonding features such as bifurcated hydrogen bonds and five-coordinated water molecules. The hydrogen-bond network has similarities to ice VI in terms of network topology and disordered hydrogen bonds. Assuming the equivalence of network components connected by pseudo symmetries, the overall network structure of this hydrate can be expressed by breaking it down into smaller structural units which correspond to the ice VI network structure. This hydrogen-bond network contains orientational disorder of water molecules in contrast to the known salt hydrates. Here, we present an example for further insights into a hydrogen-bond network containing ionic species.
Yamashita, Keishiro*; Komatsu, Kazuki*; Klotz, S.*; Fabelo, O.*; Fern ndez-D
ndez-D az, M. T.*; Abe, Jun*; Machida, Shinichi*; Hattori, Takanori; Irifune, Tetsuo*; Shimmei, Toru*; et al.
az, M. T.*; Abe, Jun*; Machida, Shinichi*; Hattori, Takanori; Irifune, Tetsuo*; Shimmei, Toru*; et al.
Proceedings of the National Academy of Sciences of the United States of America, 119(40), p.e2208717119_1 - e2208717119_6, 2022/10
Times Cited Count:2 Percentile:22.35(Multidisciplinary Sciences)Here we present the first elucidation of the disordered structure of ice VII, the dominant high-pressure form of water, at 2.2 GPa and 298 K from both single-crystal and powder neutron diffraction techniques. We reveal the three-dimensional atomic distributions from the maximum entropy method and unexpectedly find a ring-like distribution of hydrogen in contrast to the commonly-accepted discrete sites. In addition, total scattering analysis at 274 K clarified the difference in the intermolecular structure from ice VIII, the ordered counterpart of ice VII, despite an identical molecular geometry. Our complementary structure analyses robustly demonstrate the unique disordered structure of ice VII. Furthermore, these noble findings are related to the proton dynamics which drastically vary with pressure, and will contribute to an understanding of the structural origin of anomalous physical properties of ice VII under pressures.
Yamashita, Keishiro*; Komatsu, Kazuki*; Ohara, Takashi; Munakata, Koji*; Irifune, Tetsuo*; Shimmei, Toru*; Sugiyama, Kazumasa*; Kawamata, Toru*; Kagi, Hiroyuki*
High Pressure Research, 42(1), p.121 - 135, 2022/03
Times Cited Count:3 Percentile:58.88(Physics, Multidisciplinary)Komatsu, Kazuki*; Klotz, S.*; Nakano, Satoshi*; Machida, Shinichi*; Hattori, Takanori; Sano, Asami; Yamashita, Keishiro*; Irifune, Tetsuo*
High Pressure Research, 40(1), p.184 - 193, 2020/02
Times Cited Count:13 Percentile:72.32(Physics, Multidisciplinary)A new high pressure cells for neutron diffraction experiments using nano-polycrystalline anvil is presented. The cell design, off-line pressure generation tests and a gas-loading procedure for this cell are described. The performance is illustrated by powder neutron diffraction patterns of ice VII to  82 GPa. We also demonstrate the feasibility of single crystal neutron diffraction experiments of Fe
82 GPa. We also demonstrate the feasibility of single crystal neutron diffraction experiments of Fe O
O at ambient conditions using this cell and discuss the current limitation and future developments.
at ambient conditions using this cell and discuss the current limitation and future developments.
 without stacking disorder by evacuating hydrogen from hydrogen hydrate
without stacking disorder by evacuating hydrogen from hydrogen hydrateKomatsu, Kazuki*; Machida, Shinichi*; Noritake, Fumiya*; Hattori, Takanori; Sano, Asami; Yamane, Ryo*; Yamashita, Keishiro*; Kagi, Hiroyuki*
Nature Communications (Internet), 11, p.464_1 - 464_5, 2020/02
Times Cited Count:45 Percentile:87.08(Multidisciplinary Sciences)Water freezes below 0 C at ambient pressure ordinarily to ice I
C at ambient pressure ordinarily to ice I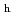 , with hexagonal stacking sequence. Under certain conditions, ice with a cubic stacking sequence can also be formed, but ideal ice I
, with hexagonal stacking sequence. Under certain conditions, ice with a cubic stacking sequence can also be formed, but ideal ice I without stacking-disorder has never been formed until recently. Here we demonstrate a route to obtain ice I
without stacking-disorder has never been formed until recently. Here we demonstrate a route to obtain ice I without stacking-disorder by degassing hydrogen from the high-pressure form of hydrogen hydrate, C
without stacking-disorder by degassing hydrogen from the high-pressure form of hydrogen hydrate, C , which has a host framework isostructural with ice I
, which has a host framework isostructural with ice I . The stacking-disorder free ice I
. The stacking-disorder free ice I is formed from C
is formed from C via an intermediate amorphous or nano-crystalline form under decompression, unlike the direct transformations occurring in ice XVI from neon hydrate, or ice XVII from hydrogen hydrate. The obtained ice I
via an intermediate amorphous or nano-crystalline form under decompression, unlike the direct transformations occurring in ice XVI from neon hydrate, or ice XVII from hydrogen hydrate. The obtained ice I shows remarkable thermal stability, until the phase transition to ice I
shows remarkable thermal stability, until the phase transition to ice I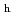 at 250 K, originating from the lack of dislocations. This discovery of ideal ice I
at 250 K, originating from the lack of dislocations. This discovery of ideal ice I will promote understanding of the role of stacking-disorder on the physical properties of ice as a counter end-member of ice I
will promote understanding of the role of stacking-disorder on the physical properties of ice as a counter end-member of ice I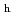 .
.
 X-ray and neutron diffraction methods
X-ray and neutron diffraction methodsYamashita, Keishiro*; Komatsu, Kazuki*; Hattori, Takanori; Machida, Shinichi*; Kagi, Hiroyuki*
Acta Crystallographica Section C; Structural Chemistry (Internet), 75(12), p.1605 - 1612, 2019/12
Times Cited Count:7 Percentile:58.9(Chemistry, Multidisciplinary)A crystal structure of a high-pressure phase of magnesium chloride hexahydrate (MgCl
 6H
6H O-II) and its deuterated counterpart (MgCl
O-II) and its deuterated counterpart (MgCl
 6D
6D O-II) have been identified for the first time by in-situ single-crystal X-ray and powder neutron diffraction. The crystal structure was analyzed by the Rietveld method for the neutron diffraction pattern based on the initial structure determined by single-crystal X-ray diffraction. This high-pressure phase has a similar framework to that in the known ambient-pressure phase, but exhibits some structural changes with symmetry reduction caused by a subtle modification in the hydrogen-bond network around the Mg(H
O-II) have been identified for the first time by in-situ single-crystal X-ray and powder neutron diffraction. The crystal structure was analyzed by the Rietveld method for the neutron diffraction pattern based on the initial structure determined by single-crystal X-ray diffraction. This high-pressure phase has a similar framework to that in the known ambient-pressure phase, but exhibits some structural changes with symmetry reduction caused by a subtle modification in the hydrogen-bond network around the Mg(H O)
O) octahedra. These structural features reflect the strain in the high-pressure phases of MgCl
octahedra. These structural features reflect the strain in the high-pressure phases of MgCl hydrates.
hydrates.