Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Pt-labeled platinum complex and evaluation of its biodistribution in healthy mice
Pt-labeled platinum complex and evaluation of its biodistribution in healthy miceOmokawa, Marina*; Kimura, Hiroyuki*; Hatsukawa, Yuichi*; Kawashima, Hidekazu*; Tsukada, Kazuaki; Yagi, Yusuke*; Naito, Yuki*; Yasui, Hiroyuki*
Bioorganic & Medicinal Chemistry, 97, p.117557_1 - 117557_6, 2024/01
Times Cited Count:0 Percentile:0.01(Biochemistry & Molecular Biology)Hidaka, Koshi*; Kimura, Toru*; Abdel-Rahman, H. M.*; Nguyen, J.-T.*; McDaniel, K. F.*; Kohlbrenner, W. E.*; Molla, A.*; Adachi, Motoyasu; Tamada, Taro; Kuroki, Ryota; et al.
Journal of Medicinal Chemistry, 52(23), p.7604 - 7617, 2009/07
Times Cited Count:19 Percentile:44.7(Chemistry, Medicinal)A series of HIV protease inhibitor based on the allophenylnorstatine structure with various P2' moieties were synthesized. Among these analogues, we discovered that a small allyl group would maintain potent enzyme inhibitory activity compared to that of the 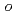 -methylbenzyl moiety in clinical candidate 1 (KNI-764, also known as JE-2147, AG-1776 or SM-319777). Introduction of an anilinic amino group to 2 (KNI-727) improved water-solubility and anti-HIV-1 activity. X-ray crystallographic analysis of 13k (KNI-1689) with a
-methylbenzyl moiety in clinical candidate 1 (KNI-764, also known as JE-2147, AG-1776 or SM-319777). Introduction of an anilinic amino group to 2 (KNI-727) improved water-solubility and anti-HIV-1 activity. X-ray crystallographic analysis of 13k (KNI-1689) with a  -methallyl group at P2' position revealed hydrophobic interactions with Ala28, Ile84, and Ile50' similar to that of 1. The presence of an additional methyl group on the allyl group in compound 13k significantly increased anti-HIV activity over 1, while providing a rational drug design for structural minimization and improving membrane permeability.
-methallyl group at P2' position revealed hydrophobic interactions with Ala28, Ile84, and Ile50' similar to that of 1. The presence of an additional methyl group on the allyl group in compound 13k significantly increased anti-HIV activity over 1, while providing a rational drug design for structural minimization and improving membrane permeability.
Adachi, Motoyasu; Ohara, Takashi; Kurihara, Kazuo; Tamada, Taro; Honjo, Eijiro; Okazaki, Nobuo; Arai, Shigeki; Shoyama, Yoshinari; Kimura, Kaname*; Matsumura, Hiroyoshi*; et al.
Proceedings of the National Academy of Sciences of the United States of America, 106(12), p.4641 - 4646, 2009/03
Times Cited Count:111 Percentile:90.72(Multidisciplinary Sciences)To further understand the catalytic mechanism and inhibitor recognition of HIV-1 protease, we need to determine the locations of key hydrogen atoms in the catalytic aspartates Asp25 and Asp125. The structure of HIV-1 protease in complex with transition-state analog KNI-272 was determined by combined neutron crystallography at 1.9  resolution and X-ray crystallography at 1.4
resolution and X-ray crystallography at 1.4  resolution. The resulting structural data shows that the catalytic residue Asp25 is protonated and that Asp125 is deprotonated. The proton on Asp25 makes a hydrogen bond with the carbonyl group of the allophenylnorstatine group in KNI-272. The deprotonated Asp125 bonds to the hydroxyl proton of Apns. The results provide direct experimental evidence for proposed aspects of the catalytic mechanism of HIV-1 protease; and can therefore contribute substantially to the development of specific inhibitors for therapeutic application.
resolution. The resulting structural data shows that the catalytic residue Asp25 is protonated and that Asp125 is deprotonated. The proton on Asp25 makes a hydrogen bond with the carbonyl group of the allophenylnorstatine group in KNI-272. The deprotonated Asp125 bonds to the hydroxyl proton of Apns. The results provide direct experimental evidence for proposed aspects of the catalytic mechanism of HIV-1 protease; and can therefore contribute substantially to the development of specific inhibitors for therapeutic application.
Matsumura, Hiroyoshi*; Adachi, Motoyasu; Sugiyama, Shigeru*; Okada, Shino*; Yamakami, Megumi*; Tamada, Taro; Hidaka, Koshi*; Hayashi, Yoshio*; Kimura, Toru*; Kiso, Yoshiaki*; et al.
Acta Crystallographica Section F, 64(11), p.1003 - 1006, 2008/11
Times Cited Count:17 Percentile:77.92(Biochemical Research Methods)This paper reports the crystallization and preliminary neutron diffraction measurements of HIV-1 protease, a potential target for anti-HIV therapy, complexed with an inhibitor (KNI-272). The aim of this neutron diffraction study is to obtain structural information about the H atoms and to determine the protonation states of the residues within the active site. The crystal was grown to a size of 1.4 mm by repeated macroseeding and a slow-cooling method using a two-liquid system. Neutron diffraction data were collected at room temperature using a BIX-4 diffractometer at the JRR-3 research reactor of the Japan Atomic Energy Agency (JAEA). The data set was integrated and scaled to 2.3
by repeated macroseeding and a slow-cooling method using a two-liquid system. Neutron diffraction data were collected at room temperature using a BIX-4 diffractometer at the JRR-3 research reactor of the Japan Atomic Energy Agency (JAEA). The data set was integrated and scaled to 2.3  resolution in space group P2(1)2(1)2, with unit-cell parameters a = 59.5, b = 87.4, c = 46.8
resolution in space group P2(1)2(1)2, with unit-cell parameters a = 59.5, b = 87.4, c = 46.8  .
.