Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 neutron diffraction study
neutron diffraction studyYamashita, Takayuki*; Koga, Norimitsu*; Mao, W.*; Gong, W.; Kawasaki, Takuro; Harjo, S.; Fujii, Hidetoshi*; Umezawa, Osamu*
Materials Science and Engineering A, 941, p.148602_1 - 148602_11, 2025/09
Cao, T.*; Wei, D.*; Gong, W.; Kawasaki, Takuro; Harjo, S.; 10 of others*
Materials Science and Engineering A, 940, p.148534_1 - 148534_16, 2025/09
Zheng, R.*; Gong, W.; 6 of others*
Acta Materialia, 293, p.121098_1 - 121098_12, 2025/07
Gu, G. H.*; Jeong, S. G.*; Heo, Y.-U.*; Harjo, S.; Gong, W.; Cho, J.*; Kim, H. S.*; 4 of others*
Journal of Materials Science & Technology, 223, p.308 - 324, 2025/07
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)Kinase, Masami
Radioisotopes, 74(2), p.233 - 238, 2025/07
no abstracts in English
Lin, Z. M.*; Liu, B. X.*; Ming, K. S.*; Xu, P. G.; Yin, F. X.*; Zheng, S. J.*
Scripta Materialia, 263, p.116692_1 - 116692_7, 2025/07
Times Cited Count:0 Percentile:0.00(Nanoscience & Nanotechnology)Mao, W.*; Gong, W.; Kawasaki, Takuro; Gao, S.*; Ito, Tatsuya; Yamashita, Takayuki*; Harjo, S.; Zhao, L.*; Wang, Q.*
Scripta Materialia, 264, p.116726_1 - 116726_6, 2025/07
Times Cited Count:0Park, M.-H.*; Shibata, Akinobu*; Harjo, S.; Tsuji, Nobuhiro*
Acta Materialia, 292, p.121061_1 - 121061_13, 2025/06
Times Cited Count:1
Shibata, Goro; 9 of others*
Applied Physics Letters, 126(24), p.241902_1 - 241902_6, 2025/06
Ohashi, Tomonori*; Sakamaki, Tatsuya*; Funakoshi, Kenichi*; Steinle-Neumann, G.*; Hattori, Takanori; Yuan, L.*; Suzuki, Akio*
Journal of Mineralogical and Petrological Sciences (Internet), 120(1), p.240926a_1 - 240926a_13, 2025/06
We explore the structures of dry and hydrated (H O and D
O and D O) Na
O) Na Si
Si O
O melt at 0-6 GPa and 1000-1300 K and glasses recovered from high pressure and temperatures by in-situ neutron and X-ray diffraction. The structures of the melts at 0-10 GPa and 3000 K are also investigated by ab-initio molecular dynamics simulation. In-situ neutron experiments revealed that the D-O distance increases with compression due to the formation of -O-D-O- bridging species, which is reproduced by the molecular dynamics simulations. The pressure-induced -O-D-O- formation reflects a more rigid incorporation of hydrogen, which acts as a mechanism for the experimentally observed higher solubility of water in silicate melts. Together with shrinking modifier domains, this process dominates the compression behavior of hydrous Na
melt at 0-6 GPa and 1000-1300 K and glasses recovered from high pressure and temperatures by in-situ neutron and X-ray diffraction. The structures of the melts at 0-10 GPa and 3000 K are also investigated by ab-initio molecular dynamics simulation. In-situ neutron experiments revealed that the D-O distance increases with compression due to the formation of -O-D-O- bridging species, which is reproduced by the molecular dynamics simulations. The pressure-induced -O-D-O- formation reflects a more rigid incorporation of hydrogen, which acts as a mechanism for the experimentally observed higher solubility of water in silicate melts. Together with shrinking modifier domains, this process dominates the compression behavior of hydrous Na Si
Si O
O melt, whereas the compression of dry Na
melt, whereas the compression of dry Na Si
Si O
O at 0-10 GPa and 3000 K is governed largely by bending of the Si-O-Si angle. The molecular dynamics simulations on hydrous Na
at 0-10 GPa and 3000 K is governed largely by bending of the Si-O-Si angle. The molecular dynamics simulations on hydrous Na Si
Si O
O melts further suggest that the sodium ions are scavenged from its network-modifying role via 2(
melts further suggest that the sodium ions are scavenged from its network-modifying role via 2(![$$^{[4]}$$](/search/images/ERPHWWZULV4SI.gif) Si-O
Si-O + Na
+ Na )
) 
![$$^{[4]}$$](/search/images/ERPHWWZULV4SI.gif) Si-(O-
Si-(O-![$$^{[5]}$$](/search/images/ERPHWWZVLV4SI.gif) Si-O)
Si-O) + 2Na
+ 2Na and Si-O
and Si-O + Na
+ Na + Si-OH
+ Si-OH  Si-(O-H-O-Si)
Si-(O-H-O-Si) + Na
+ Na with increasing pressure.
with increasing pressure.
 -nonyl N,N-dimethylammonio)-propyl sulfate, present in isotropic solutions above its upper critical solution temperature
-nonyl N,N-dimethylammonio)-propyl sulfate, present in isotropic solutions above its upper critical solution temperatureTanji, Tamao*; Kusunoki, Yuto*; Nakagawa, Taichi; Takase, Tsugiko*; Ueda, Yuki; Motokawa, Ryuhei; Hinze, W. L.*; Takagai, Yoshitaka*
Langmuir, 41(21), p.13184 - 13191, 2025/06
Times Cited Count:0Machida, Akihiko*; Saito, Hiroyuki*; Aoki, Katsutoshi*; Komatsu, Kazuki*; Hattori, Takanori; Sano, Asami; Funakoshi, Kenichi*; Machida, Shinichi*; Sato, Toyoto*; Orimo, Shinichi*
Physical Review B, 111(22), p.224413_1 - 224413_6, 2025/06
The crystal and magnetic structures of antiferromagnetic Mn deuterides formed by hydrogenating Mn metal at high temperature and high pressure, fcc  -MnDx and hcp
-MnDx and hcp 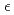 -MnDx, were investigated by in-situ neutron powder diffraction. Deuterium atoms partially occupied the octahedral interstitial positions of the fcc and hcp metal lattices. The site occupancies increased rapidly with decreasing temperature from
-MnDx, were investigated by in-situ neutron powder diffraction. Deuterium atoms partially occupied the octahedral interstitial positions of the fcc and hcp metal lattices. The site occupancies increased rapidly with decreasing temperature from  700 to
700 to  450 K and remained down to 300 K. N
450 K and remained down to 300 K. N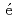 el temperature of 543(10) K was determined for
el temperature of 543(10) K was determined for  -MnD
-MnD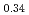 . For
. For 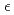 -MnD
-MnD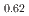 , saturation magnetic moment and N
, saturation magnetic moment and N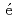 el temperature were determined to be 0.82(1)
el temperature were determined to be 0.82(1) 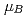 and 347(3) K, respectively. The N
and 347(3) K, respectively. The N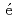 el temperatures determined for
el temperatures determined for  -MnD
-MnD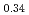 and
and 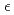 -MnD
-MnD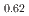 are consistent with those predicted by the respective Slater-Pauling curves proposed in previous studies. The updated N
are consistent with those predicted by the respective Slater-Pauling curves proposed in previous studies. The updated N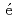 el temperatures provide insights into the development of more accurate Slater-Pauling curves based on electronic band structure calculations.
el temperatures provide insights into the development of more accurate Slater-Pauling curves based on electronic band structure calculations.
Auh, Y. H.*; Neal, N. N.*; Arole, K.*; Regis, N. A.*; Nguyen, T.*; Ogawa, Shuichi*; Tsuda, Yasutaka; Yoshigoe, Akitaka; Radovic, M.*; Green, M. J.*; et al.
ACS Applied Materials & Interfaces, 17(21), p.31392 - 31402, 2025/05
Ueda, Yuki; Micheau, C.; Motokawa, Ryuhei
Fuain Kemikaru, 54(5), p.53 - 60, 2025/05
no abstracts in English
Tomota, Yo*; Harjo, S.; Xu, P. G.; Morooka, Satoshi; Gong, W.; Wang, Y.*
Metals, 15(6), p.610_1 - 610_19, 2025/05
 Bi
Bi
Park, P.*; Ortiz, B. R.*; Spargue, M.*; Sakuya, A. P.*; Chen, S. A.*; Frontzek, M. D.*; Tian, W.*; Sibille, R.*; Mazzone, D. G.*; Tabata, Chihiro; et al.
Nature Communications (Internet), 16, p.4384_1 - 4384_9, 2025/05
Times Cited Count:0 phases in undoped and Ca-modified sodium niobates
phases in undoped and Ca-modified sodium niobatesAso, Seiyu*; Matsuo, Hiroki*; Yoneda, Yasuhiro; Morikawa, Daisuke*; Tsuda, Kenji*; Oyama, Kenji*; Ishigaki, Toru*; Noguchi, Yuji*
Physical Review B, 111(17), p.174114_1 - 174114_12, 2025/05
We investigate the crystal structures, phase transitions, and phase stability of undoped and Ca-modified NaNbO through a combined analysis of high-resolution synchrotron radiation X-ray and neutron diffraction, convergent-beam electron diffraction, and density functional theory (DFT) calculations. It is demonstrated that the antiferroelectric (AFE)-
through a combined analysis of high-resolution synchrotron radiation X-ray and neutron diffraction, convergent-beam electron diffraction, and density functional theory (DFT) calculations. It is demonstrated that the antiferroelectric (AFE)- phase is stabilized over a wide temperature range of 200 to 800 K by Ca modification, and that the NaNbO
phase is stabilized over a wide temperature range of 200 to 800 K by Ca modification, and that the NaNbO is stabilized by temperature-driven isostatic pressure accompanied by lattice expansion, whereas the Ca-modified NaNbO
is stabilized by temperature-driven isostatic pressure accompanied by lattice expansion, whereas the Ca-modified NaNbO is induced by composition-induced chemical pressure along with lattice shrinkage.
is induced by composition-induced chemical pressure along with lattice shrinkage.
 under pressure
under pressureKnafo, W.*; Thebault, T.*; Raymond, S.*; Manuel, P.*; Khalyavin, D. D.*; Orlandi, F.*; Ressouche, E.*; Beauvois, K.*; Lapertot, G.*; Kaneko, Koji; et al.
Physical Review X, 15(2), p.021075_1 - 021075_16, 2025/05
Kato, Masaru*; Zheng, J.*; Deng, Y.*; Saito, Fumie*; Unuma, Yuki*; Oka, Sayuki*; Tamura, Kazuhisa; Yagi, Ichizo*
ACS Catalysis, 15(10), p.7710 - 7719, 2025/04
Times Cited Count:0 hydridosilicate at high pressures; A Bridge to BaSiH
hydridosilicate at high pressures; A Bridge to BaSiH polyhydride
polyhydrideBeyer, D. C.*; Spektor, K.*; Vekilova, O. Y.*; Grins, J.*; Barros Brant Carvalho, P. H.*; Leinbach, L. J.*; Sannemo-Targama, M.*; Bhat, S.*; Baran, V.*; Etter, M.*; et al.
ACS Omega (Internet), 10(15), p.15029 - 15035, 2025/04
Times Cited Count:0 Percentile:0.00(Chemistry, Multidisciplinary)Hydridosilicates featuring SiH octahedral moieties represent a rather new class of compounds with potential properties relating to hydrogen storage and hydride ion conductivity. Here, we report on the new representative BaSiH
octahedral moieties represent a rather new class of compounds with potential properties relating to hydrogen storage and hydride ion conductivity. Here, we report on the new representative BaSiH obtained from reacting the Zintl phase hydride BaSiH
obtained from reacting the Zintl phase hydride BaSiH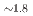 with H
with H fluid at pressures above 4 GPa and subsequent decompression to ambient pressure. It consists of complex SiH
fluid at pressures above 4 GPa and subsequent decompression to ambient pressure. It consists of complex SiH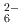 ions, which are octahedrally coordinated by Ba
ions, which are octahedrally coordinated by Ba counterions. The arrangement of Ba and Si atoms deviates only slightly from an ideal fcc NaCl structure. IR and Raman spectroscopy showed SiH
counterions. The arrangement of Ba and Si atoms deviates only slightly from an ideal fcc NaCl structure. IR and Raman spectroscopy showed SiH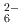 bending and stretching modes in the ranges 800-1200 and 1400-1800 cm
bending and stretching modes in the ranges 800-1200 and 1400-1800 cm , respectively. BaSiH
, respectively. BaSiH is thermally stable up to 95
is thermally stable up to 95 C above which decomposition into BaH
C above which decomposition into BaH and Si takes place. DFT calculations indicated a direct band gap of 2.5 eV. The discovery of BaSiH
and Si takes place. DFT calculations indicated a direct band gap of 2.5 eV. The discovery of BaSiH consolidates the compound class of hydridosilicates, accessible from hydrogenations of silicides at gigapascal pressures (
consolidates the compound class of hydridosilicates, accessible from hydrogenations of silicides at gigapascal pressures (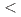 10 GPa). The structural properties of BaSiH
10 GPa). The structural properties of BaSiH suggest that it presents an intermediate (or precursor) for further hydrogenation at considerably higher pressures to the predicted superconducting polyhydride BaSiH
suggest that it presents an intermediate (or precursor) for further hydrogenation at considerably higher pressures to the predicted superconducting polyhydride BaSiH .
.