Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Rai, D.*; Yui, Mikazu; Kitamura, Akira
Progress in Nuclear Science and Technology (Internet), 5, p.19 - 26, 2018/11
The objectives of this presentation are (1) to describe the solubility method, (2) to list desirable criteria of the solubility method so that the reader can recognize which studies have been done in a way that yields quality information, (3) to present an example of how to use the evaluation criteria, and (4) to provide a few examples of future research needs where the solubility method is ideally suited and the other methods are unsuitable for these investigations.
 (am) solubility at 25
(am) solubility at 25 C in the Ca
C in the Ca -Na
-Na -H
-H -Cl
-Cl -OH
-OH -H
-H O system; A Critical review
O system; A Critical reviewRai, D.*; Kitamura, Akira; Altmaier, M.*; Rosso, K. M.*; Sasaki, Takayuki*; Kobayashi, Taishi*
Journal of Solution Chemistry, 47(5), p.855 - 891, 2018/05
Times Cited Count:8 Percentile:8.45(Chemistry, Physical)We have critically reviewed experimental data for Zr hydrolysis constant values for formation of several mononuclear and polynuclear species and a solubility product value for ZrO (am). We have determined new/revised values for the formation constants of Zr(OH)
(am). We have determined new/revised values for the formation constants of Zr(OH)
 , Zr(OH)
, Zr(OH) (aq), Zr(OH)
(aq), Zr(OH)
 , Zr(OH)
, Zr(OH)
 and Ca
and Ca Zr(OH)
Zr(OH)
 , and the solubility product for ZrO
, and the solubility product for ZrO (am) after the critical review.
(am) after the critical review.
Rai, D.*; Kitamura, Akira
Journal of Chemical Thermodynamics, 114, p.135 - 143, 2017/11
Times Cited Count:9 Percentile:19.61(Thermodynamics)Isosaccharinic acid is a cellulose degradation product that can form in low-level nuclear waste repositories and is known to form strong complexes with many elements, including actinides, disposed of in these repositories. We (1) reviewed the available data for deprotonation and lactonisation constants of isosaccharinic acid, and the isosaccharinate binding constants for Ca, Fe(III), Th, U(IV), U(VI), Np(IV), Pu(IV), and Am(III), (2) summarized complexation constant values for predicting actinide behavior in geologic repositories in the presence of isosaccharinate, and (3) outlined additional studies to acquire reliable thermodynamic data where the available data are inadequate.
 (am) in the aqueous K
(am) in the aqueous K - HCO
- HCO
 - CO
- CO
 - OH
- OH - H
- H O system
O systemRai, D.*; Kitamura, Akira; Rosso, K.*
Radiochimica Acta, 105(8), p.637 - 647, 2017/08
Times Cited Count:2 Percentile:19.65(Chemistry, Inorganic & Nuclear)Solubility of HfO (am) was determined as a function of KHCO
(am) was determined as a function of KHCO concentrations ranging from 0.001 mol.kg
concentrations ranging from 0.001 mol.kg to 0.1 mol.kg
to 0.1 mol.kg . The solubility of HfO
. The solubility of HfO (am) increased dramatically with the increase in KHCO
(am) increased dramatically with the increase in KHCO concentrations, indicating that Hf(IV) makes strong complexes with carbonate. Thermodynamic equilibrium constants for the formation of Hf-carbonate complexes were determined using both the Pitzer and SIT models. The dramatic increase in Hf concentrations with the increase in KHCO
concentrations, indicating that Hf(IV) makes strong complexes with carbonate. Thermodynamic equilibrium constants for the formation of Hf-carbonate complexes were determined using both the Pitzer and SIT models. The dramatic increase in Hf concentrations with the increase in KHCO concentrations can best be described by the formation of Hf(OH-)
concentrations can best be described by the formation of Hf(OH-) (CO
(CO )
)
 and Hf(CO
and Hf(CO )
)
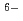 . The log
. The log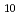 K
K values for the reactions [Hf
values for the reactions [Hf + 2 CO
+ 2 CO
 +2 OH
+2 OH
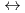 Hf(OH)
Hf(OH) (CO
(CO )
)
 ] and [Hf
] and [Hf + 5 CO
+ 5 CO

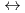 Hf(CO
Hf(CO )
)
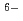 ], based on the SIT model, were determined to be 44.53
], based on the SIT model, were determined to be 44.53  0.46 and 41.53
0.46 and 41.53  0.46, respectively.
0.46, respectively.
 (cr)
(cr)Rai, D.*; Kitamura, Akira; Rosso, K. M.*; Sasaki, Takayuki*; Kobayashi, Taishi*
Radiochimica Acta, 104(8), p.583 - 592, 2016/08
Times Cited Count:5 Percentile:43.41(Chemistry, Inorganic & Nuclear)Solubility studies were conducted with HfO (cr) solid as a function of acid concentrations. These studies involved (1) using two different amounts of the solid phase, (2) acid washing the bulk solid phase, (3) preheating the solid phase to 1400
(cr) solid as a function of acid concentrations. These studies involved (1) using two different amounts of the solid phase, (2) acid washing the bulk solid phase, (3) preheating the solid phase to 1400  C, and (4) heating amorphous HfO
C, and (4) heating amorphous HfO (am) suspensions to 90
(am) suspensions to 90  C to ascertain whether the HfO
C to ascertain whether the HfO (am) converts to HfO
(am) converts to HfO (cr) and to determine the solubility from the oversaturation direction. Based on the results of these treatments it is concluded that the HfO
(cr) and to determine the solubility from the oversaturation direction. Based on the results of these treatments it is concluded that the HfO (cr) contains a small fraction of less crystalline, but not amorphous, material [HfO
(cr) contains a small fraction of less crystalline, but not amorphous, material [HfO (lcr)] and this, rather than the HfO
(lcr)] and this, rather than the HfO (cr), is the solubility-controlling phase in the range of experimental variables investigated in this study. The solubility data are interpreted using both the Pitzer and SIT models. The log
(cr), is the solubility-controlling phase in the range of experimental variables investigated in this study. The solubility data are interpreted using both the Pitzer and SIT models. The log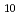 of the solubility product of HfO
of the solubility product of HfO (cr) is estimated. The observation of a small fraction of less crystalline higher solubility material is consistent with the general picture that mineral surfaces are often structurally and/or imperfect leading to a higher solubility than the bulk crystalline solid. This study stresses the urgent need, during interpretation of solubility data, of taking precautions to make certain that the observed solubility behavior for sparingly-soluble solids is assigned to the proper solid phase.
(cr) is estimated. The observation of a small fraction of less crystalline higher solubility material is consistent with the general picture that mineral surfaces are often structurally and/or imperfect leading to a higher solubility than the bulk crystalline solid. This study stresses the urgent need, during interpretation of solubility data, of taking precautions to make certain that the observed solubility behavior for sparingly-soluble solids is assigned to the proper solid phase.
 -D-isosaccharinic acid
-D-isosaccharinic acidRai, D.*; Kitamura, Akira
Journal of Nuclear Science and Technology, 53(4), p.459 - 467, 2016/04
Times Cited Count:8 Percentile:36.46(Nuclear Science & Technology)A great deal of disagreement exists in the literature regarding the intrinsic deprotonation and lactonisation constants of  -D-isosaccharinic acid (ISA). Based on a combination of nuclear magnetic resonance (NMR) and extensive experimental Ca(ISA)
-D-isosaccharinic acid (ISA). Based on a combination of nuclear magnetic resonance (NMR) and extensive experimental Ca(ISA) (cr) solubility data involving
(cr) solubility data involving  -D-isosaccharinic acid, the reliable value of log K
-D-isosaccharinic acid, the reliable value of log K for [HISA(aq)
for [HISA(aq) 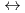 ISA
ISA + H
+ H ] is -3.27
] is -3.27  0.01 and for [HISA(aq)
0.01 and for [HISA(aq) 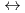 ISL(
ISL( -D-isosaccharinate-1,4-lactone)(aq) + H
-D-isosaccharinate-1,4-lactone)(aq) + H O] is 0.49
O] is 0.49  0.09. These data also provide a composite log K
0.09. These data also provide a composite log K of -3.76
of -3.76  0.09 for the reaction [ISL(aq) +H
0.09 for the reaction [ISL(aq) +H O
O 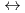 ISA
ISA + H
+ H ]. Reinterpretation of extensive Ca(ISA)
]. Reinterpretation of extensive Ca(ISA) (cr) solubility data using the SIT activity coefficient model provides log K
(cr) solubility data using the SIT activity coefficient model provides log K of -6.40
of -6.40  0.09 for [Ca(ISA)
0.09 for [Ca(ISA) (cr)
(cr) 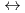 Ca
Ca + 2 (ISA)
+ 2 (ISA) ] and of 1.70
] and of 1.70  0.09 for [Ca
0.09 for [Ca + ISA
+ ISA
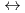 CaISA
CaISA ], based on the values that are consistent with the average of all of the available values.
], based on the values that are consistent with the average of all of the available values.
 , SO
, SO ) precipitates
) precipitatesRai, D.*; Felmy, A. R.*; Moore, D. A.*; Kitamura, Akira; Yoshikawa, Hideki; Doi, Reisuke; Yoshida, Yasushi*
Radiochimica Acta, 102(8), p.711 - 721, 2014/08
Times Cited Count:2 Percentile:16.44(Chemistry, Inorganic & Nuclear)The solubility of Ba(SeO , SO
, SO ) precipitates was determined as a function of the BaSeO
) precipitates was determined as a function of the BaSeO mole fractions, ranging from 0.0015 to 0.3830, and time with an equilibration period extending to as long as 302 days. Equilibrium/steady state conditions in this system are reached in
mole fractions, ranging from 0.0015 to 0.3830, and time with an equilibration period extending to as long as 302 days. Equilibrium/steady state conditions in this system are reached in 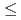 65 days. Pitzer's ion interaction model was used to calculate solid and aqueous phase activity coefficients. Thermodynamic analyses showed that the data do not satisfy Gibbs-Duhem equation, thereby demonstrating that a single-solid solution phase does not control both the selenate and sulfate concentrations. Our extensive data with [Ba], [SeO
65 days. Pitzer's ion interaction model was used to calculate solid and aqueous phase activity coefficients. Thermodynamic analyses showed that the data do not satisfy Gibbs-Duhem equation, thereby demonstrating that a single-solid solution phase does not control both the selenate and sulfate concentrations. Our extensive data with [Ba], [SeO ], and [SO
], and [SO ] can be explained with the formation of an ideal BaSeO
] can be explained with the formation of an ideal BaSeO solid solution phase that controls the selenium concentrations and a slightly disordered/less-crystalline BaSO
solid solution phase that controls the selenium concentrations and a slightly disordered/less-crystalline BaSO (s) that controls the sulfate concentrations. In these experiments the BaSO
(s) that controls the sulfate concentrations. In these experiments the BaSO component of the solid solution phase never reaches thermodynamic equilibrium with the aqueous phase. Thermodynamic interpretations of the data show that both the ideal BaSeO
component of the solid solution phase never reaches thermodynamic equilibrium with the aqueous phase. Thermodynamic interpretations of the data show that both the ideal BaSeO solid solution phase and less-crystalline BaSO
solid solution phase and less-crystalline BaSO (s) phase are in equilibrium with each other in the entire range of BaSeO
(s) phase are in equilibrium with each other in the entire range of BaSeO mole fractions investigated in this study.
mole fractions investigated in this study.
 (cr) in the aqueous Ba
(cr) in the aqueous Ba -SeO
-SeO
 -Na
-Na -H
-H -OH
-OH -H
-H O system; Extending to high selenate concentrations
O system; Extending to high selenate concentrationsRai, D.*; Felmy, A. R.*; Moore, D. A.*; Kitamura, Akira; Yoshikawa, Hideki; Doi, Reisuke; Yoshida, Yasushi*
Radiochimica Acta, 102(9), p.817 - 830, 2014/04
Times Cited Count:1 Percentile:8.88(Chemistry, Inorganic & Nuclear)The aqueous solubility of BaSeO (cr) was studied in Na
(cr) was studied in Na SeO
SeO solutions ranging in concentration from 0.0001 to 4.1 mol.kg
solutions ranging in concentration from 0.0001 to 4.1 mol.kg and maintained in a N
and maintained in a N atmosphere at room temperature (296
atmosphere at room temperature (296  2 K). The studies were conducted from both the undersaturation and oversaturation directions, with equilibration periods ranging from 3 to 596 days. The equilibrium in this system was reached rather rapidly (
2 K). The studies were conducted from both the undersaturation and oversaturation directions, with equilibration periods ranging from 3 to 596 days. The equilibrium in this system was reached rather rapidly (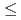 3 days). The SIT and Pitzer's ion-interaction models were used to interpret these data and the predictions based on both of these models agreed closely with the experimental data.
3 days). The SIT and Pitzer's ion-interaction models were used to interpret these data and the predictions based on both of these models agreed closely with the experimental data.
Rai, D.*; Yui, Mikazu
JAEA-Technology 2013-002, 35 Pages, 2013/05
The solubility method is one of the most powerful tools to obtain reliable thermodynamic data for (1) solubility products of discrete solids and double salts, (2) complexation constants for various ligands, (3) development of data in a wide range of pH values, (4) evaluation of data for metals that form very insoluble solids (e.g. tetravalent actinides), (5) determining solubility-controlling solids in defferent types of wastes and (6) elevated temperature for redox sensitive systems. This document is focused on describing various aspects of obtaining thermodynamic data using the solubility method. This manuscript deals with various aspects of conducting solubility studies, including selecting the study topic, modeling to define important variables, selecting the range of variables and experimental parameters, anticipating results, general equipment requirements, conducting experiments, and interpreting experimental data.