Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Pt-labeled platinum complex and evaluation of its biodistribution in healthy mice
Pt-labeled platinum complex and evaluation of its biodistribution in healthy miceOmokawa, Marina*; Kimura, Hiroyuki*; Hatsukawa, Yuichi*; Kawashima, Hidekazu*; Tsukada, Kazuaki; Yagi, Yusuke*; Naito, Yuki*; Yasui, Hiroyuki*
Bioorganic & Medicinal Chemistry, 97, p.117557_1 - 117557_6, 2024/01
Times Cited Count:0 Percentile:0.01(Biochemistry & Molecular Biology)Hidaka, Koshi*; Kimura, Toru*; Abdel-Rahman, H. M.*; Nguyen, J.-T.*; McDaniel, K. F.*; Kohlbrenner, W. E.*; Molla, A.*; Adachi, Motoyasu; Tamada, Taro; Kuroki, Ryota; et al.
Journal of Medicinal Chemistry, 52(23), p.7604 - 7617, 2009/07
Times Cited Count:19 Percentile:44.7(Chemistry, Medicinal)A series of HIV protease inhibitor based on the allophenylnorstatine structure with various P2' moieties were synthesized. Among these analogues, we discovered that a small allyl group would maintain potent enzyme inhibitory activity compared to that of the 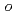 -methylbenzyl moiety in clinical candidate 1 (KNI-764, also known as JE-2147, AG-1776 or SM-319777). Introduction of an anilinic amino group to 2 (KNI-727) improved water-solubility and anti-HIV-1 activity. X-ray crystallographic analysis of 13k (KNI-1689) with a
-methylbenzyl moiety in clinical candidate 1 (KNI-764, also known as JE-2147, AG-1776 or SM-319777). Introduction of an anilinic amino group to 2 (KNI-727) improved water-solubility and anti-HIV-1 activity. X-ray crystallographic analysis of 13k (KNI-1689) with a  -methallyl group at P2' position revealed hydrophobic interactions with Ala28, Ile84, and Ile50' similar to that of 1. The presence of an additional methyl group on the allyl group in compound 13k significantly increased anti-HIV activity over 1, while providing a rational drug design for structural minimization and improving membrane permeability.
-methallyl group at P2' position revealed hydrophobic interactions with Ala28, Ile84, and Ile50' similar to that of 1. The presence of an additional methyl group on the allyl group in compound 13k significantly increased anti-HIV activity over 1, while providing a rational drug design for structural minimization and improving membrane permeability.
Adachi, Motoyasu; Ohara, Takashi; Kurihara, Kazuo; Tamada, Taro; Honjo, Eijiro; Okazaki, Nobuo; Arai, Shigeki; Shoyama, Yoshinari; Kimura, Kaname*; Matsumura, Hiroyoshi*; et al.
Proceedings of the National Academy of Sciences of the United States of America, 106(12), p.4641 - 4646, 2009/03
Times Cited Count:111 Percentile:90.72(Multidisciplinary Sciences)To further understand the catalytic mechanism and inhibitor recognition of HIV-1 protease, we need to determine the locations of key hydrogen atoms in the catalytic aspartates Asp25 and Asp125. The structure of HIV-1 protease in complex with transition-state analog KNI-272 was determined by combined neutron crystallography at 1.9  resolution and X-ray crystallography at 1.4
resolution and X-ray crystallography at 1.4  resolution. The resulting structural data shows that the catalytic residue Asp25 is protonated and that Asp125 is deprotonated. The proton on Asp25 makes a hydrogen bond with the carbonyl group of the allophenylnorstatine group in KNI-272. The deprotonated Asp125 bonds to the hydroxyl proton of Apns. The results provide direct experimental evidence for proposed aspects of the catalytic mechanism of HIV-1 protease; and can therefore contribute substantially to the development of specific inhibitors for therapeutic application.
resolution. The resulting structural data shows that the catalytic residue Asp25 is protonated and that Asp125 is deprotonated. The proton on Asp25 makes a hydrogen bond with the carbonyl group of the allophenylnorstatine group in KNI-272. The deprotonated Asp125 bonds to the hydroxyl proton of Apns. The results provide direct experimental evidence for proposed aspects of the catalytic mechanism of HIV-1 protease; and can therefore contribute substantially to the development of specific inhibitors for therapeutic application.
Matsumura, Hiroyoshi*; Adachi, Motoyasu; Sugiyama, Shigeru*; Okada, Shino*; Yamakami, Megumi*; Tamada, Taro; Hidaka, Koshi*; Hayashi, Yoshio*; Kimura, Toru*; Kiso, Yoshiaki*; et al.
Acta Crystallographica Section F, 64(11), p.1003 - 1006, 2008/11
Times Cited Count:17 Percentile:77.92(Biochemical Research Methods)This paper reports the crystallization and preliminary neutron diffraction measurements of HIV-1 protease, a potential target for anti-HIV therapy, complexed with an inhibitor (KNI-272). The aim of this neutron diffraction study is to obtain structural information about the H atoms and to determine the protonation states of the residues within the active site. The crystal was grown to a size of 1.4 mm by repeated macroseeding and a slow-cooling method using a two-liquid system. Neutron diffraction data were collected at room temperature using a BIX-4 diffractometer at the JRR-3 research reactor of the Japan Atomic Energy Agency (JAEA). The data set was integrated and scaled to 2.3
by repeated macroseeding and a slow-cooling method using a two-liquid system. Neutron diffraction data were collected at room temperature using a BIX-4 diffractometer at the JRR-3 research reactor of the Japan Atomic Energy Agency (JAEA). The data set was integrated and scaled to 2.3  resolution in space group P2(1)2(1)2, with unit-cell parameters a = 59.5, b = 87.4, c = 46.8
resolution in space group P2(1)2(1)2, with unit-cell parameters a = 59.5, b = 87.4, c = 46.8  .
.
Kuroki, Ryota
no journal, ,
X-ray and neutron crystallography enables us to obtain accurate atomic positions within proteins. The structure of porcine pancreatic elastase (PPE) with its potent inhibitor (FR130180) was determined to 0.94  resolution by X-ray diffraction and 1.75
resolution by X-ray diffraction and 1.75  resolution by neutron diffraction. It was found that there are two characteristic hydrogen bonding interactions in which hydrogen atoms were confirmed. One is located between a catalytic aspartate and histidine, another is involved in the inhibitor recognition site. The structure of HIV-PR with its potent inhibitor (KNI-272) was also determined to 0.93
resolution by neutron diffraction. It was found that there are two characteristic hydrogen bonding interactions in which hydrogen atoms were confirmed. One is located between a catalytic aspartate and histidine, another is involved in the inhibitor recognition site. The structure of HIV-PR with its potent inhibitor (KNI-272) was also determined to 0.93  resolution by X-ray diffraction and 2.3
resolution by X-ray diffraction and 2.3  resolution by neutron diffraction. The ionization state of the catalytic residues were clarified to show that Asp125 is protonated and Asp25 is deprotonated. The ionization state and the location of hydrogen atoms of the catalytic residue in HIV-PR were firstly determined by neutron diffraction.
resolution by neutron diffraction. The ionization state of the catalytic residues were clarified to show that Asp125 is protonated and Asp25 is deprotonated. The ionization state and the location of hydrogen atoms of the catalytic residue in HIV-PR were firstly determined by neutron diffraction.
Adachi, Motoyasu
no journal, ,
To understand the mechanism of interaction between HIV-1 protease and its inhibitor KNI-272, we have determined the crystal structure of HIV-1 protease in complex with KNI-272. For neutron crystallography of protein, it is needed to prepare large crystals in size using much amount of purified protein. We have used synthesized DNA optimized to express in E. coli and have purified refolded HIV-1 protease by reverse phase chromatography finally. The diffraction data were collected using BIX-4 at JRR-3 in JAEA. The structure was refined by program PHENIX. Our results indicates that the carbonyl group of allophenylnorstatine (Apns) in KNI-272 forms a significant hydrogen bond with protonated Asp 25, and the hydrogen atom from the hydroxyl group of Apns forms a remarkable hydrogen bond with the deprotonated Asp125. These results show direct evidence that Asp125 provides a proton to carbonyl group of substrate and Asp25 contributes to activate the attacking water molecule as a nucleophile.
Adachi, Motoyasu; Ohara, Takashi; Kurihara, Kazuo; Tamada, Taro; Honjo, Eijiro; Okazaki, Nobuo; Arai, Shigeki; Shoyama, Yoshinari; Matsumura, Hiroyoshi*; Sugiyama, Shigeru*; et al.
no journal, ,
We have determined a crystal structure of HIV-1 protease by neutron crystallography. The development of HIV-1 protease inhibitors is regarded as a major success of structure-based drug design and contributes to establish highly active anti-retroviral therapy for AIDS. To further understand the catalytic mechanism of HIV-1 protease and interaction between HIV-1 protease and its inhibitor, we have determined the crystal structure of HIV-1 protease in complex with a inhibitor, KNI-272 to 2.3  resolution by neutron crystallography. Our results indicates that the carbonyl group of allophenylnorstatine (Apns) in KNI-272 forms a significant hydrogen bond with protonated Asp 25, and the hydrogen atom from the hydroxyl group of Apns forms a remarkable hydrogen bond with the deprotonated Asp125. These results show direct evidence that Asp25 provides a proton to carbonyl group of substrate and Asp125 contributes to activate the attacking water molecule as a nucleophile.
resolution by neutron crystallography. Our results indicates that the carbonyl group of allophenylnorstatine (Apns) in KNI-272 forms a significant hydrogen bond with protonated Asp 25, and the hydrogen atom from the hydroxyl group of Apns forms a remarkable hydrogen bond with the deprotonated Asp125. These results show direct evidence that Asp25 provides a proton to carbonyl group of substrate and Asp125 contributes to activate the attacking water molecule as a nucleophile.
Adachi, Motoyasu; Ohara, Takashi; Kurihara, Kazuo; Tamada, Taro; Honjo, Eijiro; Okazaki, Nobuo; Arai, Shigeki; Shoyama, Yoshinari; Kimura, Kaname*; Matsumura, Hiroyoshi*; et al.
no journal, ,
HIV-1 protease is a dimeric aspartic protease that cleaves the nascent polyproteins of HIV-1 and plays an essential role in viral replication. To further understand the catalytic mechanism of HIV-1 protease, we have determined the crystal structure of HIV-1 protease in complex with a transition state mimetic tripeptide inhibitor, KNI-272 to 1.9  resolution by neutron crystallography in combination with 1.4
resolution by neutron crystallography in combination with 1.4  resolution X-ray diffraction data. Our results indicates that the carbonyl group of allophenylnorstatine in KNI-272 forms a significant hydrogen bond with protonated Asp 25, and the hydrogen atom from the hydroxyl group of Apns forms a remarkable hydrogen bond with the deprotonated Asp125. These results show direct evidence that Asp25 provides a proton to carbonyl group of substrate and Asp125 contributes to activate the attacking water molecule as a nucleophile.
resolution X-ray diffraction data. Our results indicates that the carbonyl group of allophenylnorstatine in KNI-272 forms a significant hydrogen bond with protonated Asp 25, and the hydrogen atom from the hydroxyl group of Apns forms a remarkable hydrogen bond with the deprotonated Asp125. These results show direct evidence that Asp25 provides a proton to carbonyl group of substrate and Asp125 contributes to activate the attacking water molecule as a nucleophile.
Midorikawa, Masamichi*; Yamashita, Shinichi; Katsumura, Yosuke; Lin, M.; Muroya, Yusa*; Maeyama, Takuya*; Funtowiez, D.*; Kamibayashi, Masato*; Anzai, Kazunori*
no journal, ,
no abstracts in English
Tamada, Taro; Ohara, Takashi; Kurihara, Kazuo; Kuroki, Ryota
no journal, ,
no abstracts in English
Midorikawa, Masamichi*; Oka, Toshitaka; Yamashita, Shinichi; Muroya, Yusa*; Lin, M.; Kamibayashi, Masato*; Anzai, Kazunori*; Kudo, Hisaaki*; Katsumura, Yosuke
no journal, ,
5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1pyrroline N-oxide (CYPMPO) is a recently developed spin-trapping agent, which is expected to distinguish O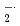 from
from  OH. We have determined its reactivity with water decomposed radicals, especially with
OH. We have determined its reactivity with water decomposed radicals, especially with  OH and with e
OH and with e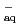 by pulse radiolysis, and compared results with commonly-used spin trapping agent, 5,5-dimethyl-1-pyrroline N-oxide (DMPO). Furthermore, the reactions with oxidative radicals such as
by pulse radiolysis, and compared results with commonly-used spin trapping agent, 5,5-dimethyl-1-pyrroline N-oxide (DMPO). Furthermore, the reactions with oxidative radicals such as  COO
COO , NO
, NO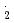 and Br
and Br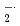 have also been investigated.
have also been investigated.
Adachi, Motoyasu; Ohara, Takashi; Kurihara, Kazuo; Tamada, Taro; Honjo, Eijiro; Okazaki, Nobuo; Arai, Shigeki; Shoyama, Yoshinari; Matsumura, Hiroyoshi*; Sugiyama, Shigeru*; et al.
no journal, ,
no abstracts in English
Adachi, Motoyasu; Arai, Shigeki; Tamada, Taro; Kuroki, Ryota; Hidaka, Koshi*; Kimura, Toru*; Kiso, Yoshiaki*
no journal, ,
To obtain information for design of inhibitor against drug resistant mutant HIV protease, we determined X-ray crystal structures of A17 type HIV-1 protease complexed with inhibitors of lopinavir and KNI-1657. The gene of A17 type HIV-1 protease was synthesized chemically, and prepared using E. coli expression system. The results showed that affinity of lopinavir to the protease was 700 times lower than that of wild-type, and KNI-1657 has 20 times higher affinity to the protease than lopinavir. The crystals of complex were obtained using PEG4000 as precipitant. The diffraction data were collected at PF and SPring-8, and the structures were refined at R-factor of 19%.
Adachi, Motoyasu; Ohara, Takashi; Kurihara, Kazuo; Tamada, Taro; Honjo, Eijiro*; Okazaki, Nobuo; Arai, Shigeki; Shoyama, Yoshinari*; Matsumura, Hiroyoshi*; Adachi, Hiroaki*; et al.
no journal, ,
To understand the catalytic mechanism of HIV-1 protease, we have determined the crystal structure of HIV-1 protease in complex with a transition state mimetic inhibitor, KNI-272 by neutron crystallography. Our results indicates that the carbonyl group of allophenylnorstatine in KNI-272 forms a significant hydrogen bond with protonated Asp 25, and the hydrogen atom from the hydroxyl group of allophenylnorstatine forms a remarkable hydrogen bond with the deprotonated Asp125. These results show direct evidence that Asp25 provides a proton to carbonyl group of substrate and Asp125 contributes to activate the attacking water molecule as a nucleophile.
Adachi, Motoyasu; Ohara, Takashi; Kurihara, Kazuo; Tamada, Taro; Honjo, Eijiro*; Okazaki, Nobuo; Arai, Shigeki; Shoyama, Yoshinari*; Matsumura, Hiroyoshi*; Sugiyama, Shigeru*; et al.
no journal, ,
In this study, we determined crystal structures of HIV-1 protease complexed with inhibitor by neutron and X-ray crystallography. Finally, we refined the structures to R-factor of 17.3% and free R-factor 20.3% by neutron crystallography and to R-factor of 10.4 % and free R-factor 12.4% by X-ray crystallography. The result shows that Asp 25 residue is protonated and Asp 125 is deprotonated. These information is important to resolve catalytic mechanism and design of new potent inhibitor.
Adachi, Motoyasu; Ohara, Takashi; Kurihara, Kazuo; Tamada, Taro; Honjo, Eijiro*; Okazaki, Nobuo; Arai, Shigeki; Shoyama, Yoshinari*; Matsumura, Hiroyoshi*; Adachi, Hiroaki*; et al.
no journal, ,
HIV-1 protease is a dimeric aspartic protease that cleaves the nascent polyproteins of HIV-1 and plays an essential role in viral replication. To further understand the catalytic mechanism of HIV-1 protease, we have determined the crystal structure of HIV-1 protease in complex with a transition state mimetic tripeptide inhibitor, KNI-272 to 1.9  ; resolution by neutron crystallography in combination with 1.4
; resolution by neutron crystallography in combination with 1.4  ; resolution X-ray diffraction data. Our results indicates that the carbonyl group of allophenylnorstatine (Apns) in KNI-272 forms a significant hydrogen bond with protonated Asp 25, and the hydrogen atom from the hydroxyl group of Apns forms a remarkable hydrogen bond with the deprotonated Asp125. These results show direct evidence that Asp25 provides a proton to carbonyl group of substrate and Asp125 contributes to activate the attacking water molecule as a nucleophile.
; resolution X-ray diffraction data. Our results indicates that the carbonyl group of allophenylnorstatine (Apns) in KNI-272 forms a significant hydrogen bond with protonated Asp 25, and the hydrogen atom from the hydroxyl group of Apns forms a remarkable hydrogen bond with the deprotonated Asp125. These results show direct evidence that Asp25 provides a proton to carbonyl group of substrate and Asp125 contributes to activate the attacking water molecule as a nucleophile.
Adachi, Motoyasu; Tamada, Taro; Kuroki, Ryota; Hidaka, Koshi*; Kimura, Toru*; Kiso, Yoshiaki*; Yamamoto, Kohei*; Kidokoro, Shunichi*
no journal, ,
no abstracts in English
Hidaka, Koshi*; Adachi, Motoyasu; Kuroki, Ryota; Tokai, Satoko*; Akaji, Kenichi*; Tsuda, Yuko*; Kiso, Yoshiaki*
no journal, ,
In the HIV protease inhibitor study, we faced on a problem of much difference in activity of a compound with 2,6-dimethylphenoxyacetyl moiety against the enzyme and the virus. The protease inhibitory potency was plateau because of the limited structural modifications. Therefore, we shifted to modify the property such as reduction of the hydrophobicity. During the struggles, amino substitution succeeded in improving the water solubility to enhance the anti-HIV activity and with the sustained protease inhibitory activity. This result stimulated us to modify the Apns-containing inhibitors for the development of therapeutic drug and to utilize them as aspartic proteases probes.
Hidaka, Koshi*; Toda, Yuki*; Adachi, Motoyasu; Kuroki, Ryota; Kiso, Yoshiaki*
no journal, ,
Molecular dynamic simulations of the inhibitor suggested existence of additional stable bridging water molecules to support the binding with mutated proteases. To increase the numbers of bridging water molecules, we replaced amide with sulfonyl and oxamide structures at both terminals of pseudo-symmetric peptides based on HMC. In summary, oxamide modifications resulted in a moderate activity loss against lopinavir-resistant mutations compared to inhibitors without oxamide. This method to accompany multiple bridging water molecules could be applicable to design protease inhibitors to defeat the drug resistance from amino acid mutations.