Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Np
Np )O
)O
Otobe, Haruyoshi; Akabori, Mitsuo; Arai, Yasuo; Minato, Kazuo
Journal of the American Ceramic Society, 92(1), p.174 - 178, 2009/01
Times Cited Count:7 Percentile:42.38(Materials Science, Ceramics)The oxygen potentials of the oxygen-deficient fluorite-type oxide Am Pu
Pu O
O were measured by the electrochemical method with using a zirconia solid-electrolyte. The coulomb titration has been made for the sample at 1333 K over 0.02
were measured by the electrochemical method with using a zirconia solid-electrolyte. The coulomb titration has been made for the sample at 1333 K over 0.02 

 0.25. The oxygen potentials were -93.63 and -440.18 kJmol
0.25. The oxygen potentials were -93.63 and -440.18 kJmol for
for  = 0.021 and 0.25 at 1333 K, respectively. The temperature dependence of the oxygen potentials was also measured between 1000 and 1333 K over the
= 0.021 and 0.25 at 1333 K, respectively. The temperature dependence of the oxygen potentials was also measured between 1000 and 1333 K over the  range of 0.02
range of 0.02 

 0.243. The temperature dependence was almost linear over the
0.243. The temperature dependence was almost linear over the  and temperature ranges concerned.
and temperature ranges concerned.
Otobe, Haruyoshi; Akabori, Mitsuo; Minato, Kazuo
Journal of the American Ceramic Society, 91(6), p.1981 - 1985, 2008/06
Times Cited Count:20 Percentile:70.46(Materials Science, Ceramics)The oxygen potentials of AmO were measured in the
were measured in the  range of 0.01 to 0.5 and the temperature range of 1000 to 1333 K by the electromotive force (EMF) method. The oxygen potentials at 1333 K were -19.83 kJ/mol for
range of 0.01 to 0.5 and the temperature range of 1000 to 1333 K by the electromotive force (EMF) method. The oxygen potentials at 1333 K were -19.83 kJ/mol for  =0.01 and -319.1 kJ/mol for
=0.01 and -319.1 kJ/mol for  =0.485, which were higher than those of CeO
=0.485, which were higher than those of CeO by approximately 200 kJ/mol for the corresponding
by approximately 200 kJ/mol for the corresponding  values. From the dependence of the oxygen potentials on
values. From the dependence of the oxygen potentials on  and temperature, a tentative phase diagram of Am-O system was proposed, which suggested the presence of the intermediate phases of Am
and temperature, a tentative phase diagram of Am-O system was proposed, which suggested the presence of the intermediate phases of Am O
O and Am
and Am O
O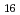 in the Am-O system.
in the Am-O system.
Hayashi, Hirokazu; Takano, Masahide; Akabori, Mitsuo; Minato, Kazuo
Journal of Alloys and Compounds, 456(1-2), p.243 - 246, 2008/05
Times Cited Count:8 Percentile:45.97(Chemistry, Physical)Americium trichloride was synthesized by the reaction of americium nitride with cadmium chloride at 600-660 K in a dynamic vacuum. The product was hexagonal AmCl , of which lattice parameters were determined to be
, of which lattice parameters were determined to be 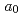 = 0.7390 and
= 0.7390 and 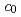 = 0.4215 nm. The results indicate that high purity AmCl
= 0.4215 nm. The results indicate that high purity AmCl samples, in which the oxychloride was not found, were prepared without the use of corrosive reagents. The reaction of the nitrides with cadmium chloride is suitable for synthesis of high purity actinide and lanthanide chlorides.
samples, in which the oxychloride was not found, were prepared without the use of corrosive reagents. The reaction of the nitrides with cadmium chloride is suitable for synthesis of high purity actinide and lanthanide chlorides.
Otobe, Haruyoshi; Akabori, Mitsuo; Arai, Yasuo; Minato, Kazuo
Proceedings of 3rd International ATALANTE Conference (ATALANTE 2008) (CD-ROM), 4 Pages, 2008/05
The oxygen potentials of cubic zirconia containing Pu and americia have been measured by the electromotive force (EMF) method with a zirconia solid-electrolyte. The oxygen potentials of these oxides were reviewed. The phase relations, microstructure, equilibrium state of these oxides were discussed, referring to the isothermal curve of the oxygen potentials. It was found that the oxygen potentials are sensitive to the phase transitions. Moreover, the study on the microstructure around an element incorporated in oxide fuels as well as its phase is necessary to predict the effect of the element on the oxygen potentials of oxide fuels.

Nishi, Tsuyoshi; Takano, Masahide; Ito, Akinori; Akabori, Mitsuo; Arai, Yasuo; Minato, Kazuo; Numata, Masami
Journal of Nuclear Materials, 373(1-3), p.295 - 298, 2008/02
Times Cited Count:16 Percentile:70.82(Materials Science, Multidisciplinary)The thermal diffusivity of americium oxide was determined in the temperature range from 299 to 1473 K by a laser flash method. The thermal diffusivity of AmO decreased with increasing temperature. The thermal conductivity of AmO
decreased with increasing temperature. The thermal conductivity of AmO was estimated from the measured thermal diffusivity, the specific heat capacity and the bulk density. It was found that the thermal conductivity of AmO
was estimated from the measured thermal diffusivity, the specific heat capacity and the bulk density. It was found that the thermal conductivity of AmO decreased with increasing temperature over the temperature range investigated. It was also found that the decrease in O/Am ratio during the thermal diffusivity measurements under vacuum resulted in a slight decrease in thermal conductivity of AmO
decreased with increasing temperature over the temperature range investigated. It was also found that the decrease in O/Am ratio during the thermal diffusivity measurements under vacuum resulted in a slight decrease in thermal conductivity of AmO .
.
Sugikawa, Susumu; Nakazaki, Masato; Kimura, Akihiro; Kida, Takashi*; Kihara, Takehiro*; Akabori, Mitsuo; Minato, Kazuo; Suda, Kazuhiro*; Chikazawa, Takahiro*
Nihon Genshiryoku Gakkai Wabun Rombunshi, 6(4), p.476 - 483, 2007/12
A one-step simple extraction chromatography method using TODGA (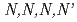 -tetraoctyl-diglycolamide) adsorbent column has been developed to separate the americium from plutonium-solvent extraction raffinate. The raffinate contained Am(
-tetraoctyl-diglycolamide) adsorbent column has been developed to separate the americium from plutonium-solvent extraction raffinate. The raffinate contained Am( 620 mg/
620 mg/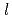 ), Np(
), Np( 107 mg/
107 mg/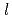 ), Ag(
), Ag( 2000 mg/
2000 mg/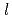 ), Fe(
), Fe( 290 mg/
290 mg/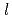 ), Cr(
), Cr( 38 mg/
38 mg/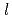 ), Ni(
), Ni( 52 mg/
52 mg/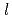 ) and trace of TBP. Small-scale and scale-up tests for separation of americium and conversion to americium oxide were carried out in NUCEF. Efforts were made to increase yield and purity of americium. The americium was separated with 83-92% yields and 97-98% purities by small-scale tests and 85-95% yields and 98-99% purities by scale-up tests. The yields for conversion of americium nitrate solution to americium oxide were 89-100% by small-scale tests and 85-96 % by scale-up tests. Approximately 1.8 gram americium oxide was recovered from 6 litres of the raffinate and supplied for the research on the high-temperature chemistry of TRU.
) and trace of TBP. Small-scale and scale-up tests for separation of americium and conversion to americium oxide were carried out in NUCEF. Efforts were made to increase yield and purity of americium. The americium was separated with 83-92% yields and 97-98% purities by small-scale tests and 85-95% yields and 98-99% purities by scale-up tests. The yields for conversion of americium nitrate solution to americium oxide were 89-100% by small-scale tests and 85-96 % by scale-up tests. Approximately 1.8 gram americium oxide was recovered from 6 litres of the raffinate and supplied for the research on the high-temperature chemistry of TRU.
Hayashi, Hirokazu; Akabori, Mitsuo; Minato, Kazuo; Mizuguchi, Koji*; Kawabe, Akihiro*; Fujita, Reiko*
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 75(7), p.528 - 534, 2007/07
Times Cited Count:0 Percentile:0.01(Electrochemistry)The simulation code for the pyrochemical processing of spent nuclear fuels was developed to analyze experimental data, to predict experimental results, and to propose adequate conditions and processes. The Simulation code for Pyrochemical Reprocessing (SPR) is based on calculations of chemical equilibrium and electrochemical reactions. The code also includes the calculations of the current-potential distribution between the electrodes. Some calculations were made to simulate the experimental results on the electro-codeposition process of UO and PuO
and PuO . The phenomena of the redox reactions between Pu
. The phenomena of the redox reactions between Pu and Pu
and Pu ions and those between Fe
ions and those between Fe and Fe
and Fe ions were theoretically analyzed; these redox reactions cause the low current efficiency in the electro-codeposition process. The calculated current-potential distribution around the cathode corresponds to the observed distribution of the oxide deposited on the cathode.
ions were theoretically analyzed; these redox reactions cause the low current efficiency in the electro-codeposition process. The calculated current-potential distribution around the cathode corresponds to the observed distribution of the oxide deposited on the cathode.
Yamagishi, Hideshi; Kakuta, Tsunemi*
Kyodo Kenkyu Seika Gaiyo, Shoraigata Genshiryoku Hatsuden Gijutsu No Kodoka Ni Kansuru Kenkyu, p.197 - 211, 2006/06
no abstracts in English
Hayashi, Hirokazu; Akabori, Mitsuo; Minato, Kazuo
Proceedings of International Conference on Nuclear Energy System for Future Generation and Global Sustainability (GLOBAL 2005) (CD-ROM), 3 Pages, 2005/10
For a basis of the future nuclear cycle, it is very important to understand and control the behavior of TRU (Np, Pu, Am, Cm) in the nuclear fuel cycle. Experimental study of pyrochemical process of fuels containing TRU requires the facility having not only shielding for  -ray and neutron but also ability to keep a high purity inert gas atmosphere; because minor actinide chlorides can easily react with oxygen or water vapor in an atmosphere. The module for TRU high temperature chemistry (TRU-HITEC) had been installed to study the basic properties of TRU in the pyrochemical processes. In the present work, the behavior of
-ray and neutron but also ability to keep a high purity inert gas atmosphere; because minor actinide chlorides can easily react with oxygen or water vapor in an atmosphere. The module for TRU high temperature chemistry (TRU-HITEC) had been installed to study the basic properties of TRU in the pyrochemical processes. In the present work, the behavior of  Am in pyrochemical process was investigated by electrochemical methods.
Am in pyrochemical process was investigated by electrochemical methods.
Minato, Kazuo; Akabori, Mitsuo; Tsuboi, Takashi; Kurobane, Shiro; Hayashi, Hirokazu; Takano, Masahide; Otobe, Haruyoshi; Misumi, Masahiro*; Sakamoto, Takuya*; Kato, Isao*; et al.
JAERI-Tech 2005-059, 61 Pages, 2005/09
An experimental facility called the Module for TRU High Temperature Chemistry (TRU-HITEC) was installed in the Back-end Cycle Key Elements Research Facility (BECKY) of the Nuclear Fuel Cycle Safety Engineering Research Facility (NUCEF) for the basic studies of the behavior of the transuranium elements (TRU) in pyrochemical reprocessing and oxide fuels. TRU-HITEC consists of three alpha/gamma cells shielded by steel and polyethylene and a glove box shielded by leaded acrylic resin, where experimental apparatuses have been equipped and a high purity argon gas atmosphere is maintained. In the facility 10 g of  Am as well as the other TRU of Np, Pu and Cm can be handled. This report summarizes the outline, structure, performance and interior apparatuses of the facility, and is the result of the joint research between the Japan Atomic Energy Research Institute and three electric power companies of Tokyo Electric Power Co., Tohoku Electric Power Co. and the Japan Atomic Power Co.
Am as well as the other TRU of Np, Pu and Cm can be handled. This report summarizes the outline, structure, performance and interior apparatuses of the facility, and is the result of the joint research between the Japan Atomic Energy Research Institute and three electric power companies of Tokyo Electric Power Co., Tohoku Electric Power Co. and the Japan Atomic Power Co.
The Working Team for Examination of the Sample from Core Shrouds and Primary Loop Recirculation Pipi
JAERI-Tech 2004-012, 62 Pages, 2004/02
At Onagawa Nuclear Power Station Unit-1 of the Tohoku Electric Power co., inc., cracks were confirmed near welded joints of core shroud in 15th periodical inspection. Tohoku Electric Power co., inc. has conducted a material examination with Nippon Nuclear Fuel Development Co., Ltd.. To investigate independently, a JAERI's own evaluation report was provided. The results are as follows; (1) Hardening layer was detected at the depth of about 150-250 m from outer surface of the sample. (2) Corrosion products were observed on inner surface of the cracks and some of them penetrated into grains. (3) Transgranular cracking and intergranular cracking were observed at the region within about 100
m from outer surface of the sample. (2) Corrosion products were observed on inner surface of the cracks and some of them penetrated into grains. (3) Transgranular cracking and intergranular cracking were observed at the region within about 100 m and the deeper region more than about 200
m and the deeper region more than about 200 m in depth from outer surface of the sample, respectively. (4) Distinct chromium depletion was not detected at the grain boundaries. (5) Chemical compositions of the sample corresponded to type 304L stainless steel in Japanese Industrial Standard. From the above, it is concluded that the cracks are stress corrosion cracking.
m in depth from outer surface of the sample, respectively. (4) Distinct chromium depletion was not detected at the grain boundaries. (5) Chemical compositions of the sample corresponded to type 304L stainless steel in Japanese Industrial Standard. From the above, it is concluded that the cracks are stress corrosion cracking.
Yamagishi, Hideshi; Soyama, Kazuhiko; Kakuta, Tsunemi; Ochiai, Masaaki; Iwamura, Takamichi; Saishu, Sadanori*; Urakami, Masao*; Masuda, Naohiro*; Yamauchi, Yuki*; Otani, Junichi*; et al.
JAERI-Tech 2001-053, 19 Pages, 2001/08
no abstracts in English
Nishi, Tsuyoshi; Takano, Masahide; Ito, Akinori; Akabori, Mitsuo; Minato, Kazuo; Kizaki, Minoru
no journal, ,
no abstracts in English

Otobe, Haruyoshi; Akabori, Mitsuo; Minato, Kazuo
no journal, ,
The oxygen potentials of oxygen-deficient fluorite-type oxide (Am,Np)O have been measured by the electrochemical method with using the zirconia solid-electrolyte. The coulomb titration has been made for the sample at the measured temperature of 1333 K over 0.01
have been measured by the electrochemical method with using the zirconia solid-electrolyte. The coulomb titration has been made for the sample at the measured temperature of 1333 K over 0.01

 0.25 to clarify the relation between the oxygen potentials and oxygen deficiency
0.25 to clarify the relation between the oxygen potentials and oxygen deficiency  . The measured temperature has also been changed from 1333 to 1000 K at the constant rate of 0.5 K/minute at the constant
. The measured temperature has also been changed from 1333 to 1000 K at the constant rate of 0.5 K/minute at the constant  to clarify the temperature dependency of the oxygen potentials. The oxygen potentials have been -90 and -380 kJ/mol at
to clarify the temperature dependency of the oxygen potentials. The oxygen potentials have been -90 and -380 kJ/mol at  =0.01 and 0.24 at 1333 K, reflectivily. The temperature dependency of the oxygen potentials showed an almost straight line between 1333 and 1100 K, whereas it had a curved line and fluctuation below 1100 K.
=0.01 and 0.24 at 1333 K, reflectivily. The temperature dependency of the oxygen potentials showed an almost straight line between 1333 and 1100 K, whereas it had a curved line and fluctuation below 1100 K.
Hayashi, Hirokazu; Akabori, Mitsuo; Minato, Kazuo; Mizuguchi, Koji*; Kawabe, Akihiro*; Fujita, Reiko*
no journal, ,
The simulation code for the pyrochemical processing of spent nuclear fuels was developed to analyze experimental data, to predict experimental results, and to propose adequate conditions and processes. The Simulation code for Pyrochemical Reprocessing (SPR) is based on calculations of chemical equilibrium and electrochemical reactions. The code also includes the calculations of the current-potential distribution between the electrodes. Some calculations were made to simulate the experimental results on the electro-codeposition process of UO and PuO
and PuO . The phenomena of the redox reactions between Pu
. The phenomena of the redox reactions between Pu and Pu
and Pu ions and those between Fe
ions and those between Fe and Fe
and Fe ions were theoretically analyzed; these redox reactions cause the low current efficiency in the electro-codeposition process. The calculated current-potential distribution around the cathode corresponds to the observed distribution of the oxide deposited on the cathode.
ions were theoretically analyzed; these redox reactions cause the low current efficiency in the electro-codeposition process. The calculated current-potential distribution around the cathode corresponds to the observed distribution of the oxide deposited on the cathode.
Hayashi, Hirokazu; Minato, Kazuo*
no journal, ,
For a basis of the future nuclear fuel cycle, understanding the behavior of transuranium elements (TRU: Np, Pu, Am, Cm) is important. Experimental study on pyrochemical reprocessing of the fuels containing TRU is one of the important topics. But limited data are available on the behavior of TRU in some molten salts including NaCl-2CsCl melt which is considered to be used for pyrochemical reprocessing of MOX fuels in RIAR process. Only the electrochemical behavior of Np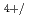 Np
Np redox couple has been reported for Np ions in NaCl-2CsCl melt. In the present study, behavior of Np ions including the reduction of Np
redox couple has been reported for Np ions in NaCl-2CsCl melt. In the present study, behavior of Np ions including the reduction of Np in NaCl-2CsCl melt was investigated. A typical cyclic voltammogram shows 2 groups of the signals corresponding to Np species. They were assigned to the redox reactions of Np
in NaCl-2CsCl melt was investigated. A typical cyclic voltammogram shows 2 groups of the signals corresponding to Np species. They were assigned to the redox reactions of Np /Np and Np
/Np and Np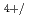 Np
Np , respectively by comparing with those observed in LiCl-KCl melt. The redox potentials and the diffusion coefficients obtained by various transient electrochemical techniques will be presented.
, respectively by comparing with those observed in LiCl-KCl melt. The redox potentials and the diffusion coefficients obtained by various transient electrochemical techniques will be presented.