Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ohira, Saki; Abe, Takeyasu; Iida, Yoshihisa
Radiochimica Acta, 111(7), p.525 - 531, 2023/07
Times Cited Count:0 Percentile:0.01(Chemistry, Inorganic & Nuclear)The solubility of 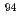 Nb in calcium alkaline solutions is one of the important parameters in safety assessment of intermediate-depth disposal which are assumed to use cementitious materials. Nb solubility and solubility-limiting solid phases of Nb in these systems remain unclear. The oversaturation solubility experiments were performed systematically in the 0.001-0.1 M CaCl
Nb in calcium alkaline solutions is one of the important parameters in safety assessment of intermediate-depth disposal which are assumed to use cementitious materials. Nb solubility and solubility-limiting solid phases of Nb in these systems remain unclear. The oversaturation solubility experiments were performed systematically in the 0.001-0.1 M CaCl solutions under alkali conditions, and the characterization of precipitated solid phase controlling Nb solubility was conducted. The negative dependence of Nb solubilities on pH and Ca concentration was observed in solubility experiments, the molar ratio of Nb to Ca of precipitated solid phase was 0.66. The pH and Ca dependence of Nb solubilities was reproduced by the reaction with Nb aqueous species Nb(OH)
solutions under alkali conditions, and the characterization of precipitated solid phase controlling Nb solubility was conducted. The negative dependence of Nb solubilities on pH and Ca concentration was observed in solubility experiments, the molar ratio of Nb to Ca of precipitated solid phase was 0.66. The pH and Ca dependence of Nb solubilities was reproduced by the reaction with Nb aqueous species Nb(OH)
 and Ca-Nb oxide with the molar ratio of Nb to Ca 0.66, e.g., Ca
and Ca-Nb oxide with the molar ratio of Nb to Ca 0.66, e.g., Ca Nb
Nb O
O (am).
(am).
Ohira, Saki; Iida, Yoshihisa
Proceedings of Waste Management Symposia 2023 (WM2023) (Internet), 10 Pages, 2023/02
The sorption distribution coefficient ( d) of niobium-94 (Nb-94) on minerals is one of the important parameters in safety assessment of radioactive waste disposal. In a previous study, the
d) of niobium-94 (Nb-94) on minerals is one of the important parameters in safety assessment of radioactive waste disposal. In a previous study, the  d values of Nb under alkali condition in the presence of Ca, were two orders of magnitude higher than those in the presence of Na. In this study, Nb sorption experiments were performed to reexamine the effect of Ca on Nb sorption on clay minerals, and blank tests were performed to check for precipitation formation. The results showed that the Nb sorption onto montmorillonite and illite, did not depend on the Ca concentration, and
d values of Nb under alkali condition in the presence of Ca, were two orders of magnitude higher than those in the presence of Na. In this study, Nb sorption experiments were performed to reexamine the effect of Ca on Nb sorption on clay minerals, and blank tests were performed to check for precipitation formation. The results showed that the Nb sorption onto montmorillonite and illite, did not depend on the Ca concentration, and  d values obtained in the presence of Ca were the same as those in the absence of Ca. A sorption model assuming sorption by complexation on the mineral surface was developed and then calculated using the geochemical calculation code. The model with the surface species X_ONb(OH)
d values obtained in the presence of Ca were the same as those in the absence of Ca. A sorption model assuming sorption by complexation on the mineral surface was developed and then calculated using the geochemical calculation code. The model with the surface species X_ONb(OH) and X_ONb(OH)
and X_ONb(OH)
 represented trends in the data obtained.
represented trends in the data obtained.
 Cs/
Cs/ Cs isotopic ratio in soil collected near Fukushima Daiichi Nuclear Power Station through mass spectrometry
Cs isotopic ratio in soil collected near Fukushima Daiichi Nuclear Power Station through mass spectrometryShimada, Asako; Tsukahara, Takehiko*; Nomura, Masao*; Kim, M. S.*; Shimada, Taro; Takeda, Seiji; Yamaguchi, Tetsuji
Journal of Nuclear Science and Technology, 58(11), p.1184 - 1194, 2021/11
Times Cited Count:5 Percentile:65.59(Nuclear Science & Technology)Determining the completeness of nuclear reactor decommissioning is an important step in safely utilizing nuclear power. For example,  Cs from the Fukushima Daiichi Nuclear Power Station (FDNPS) accident can be treated as background radioactivity, so determining the origin of
Cs from the Fukushima Daiichi Nuclear Power Station (FDNPS) accident can be treated as background radioactivity, so determining the origin of  Cs is essential. To accomplish this, measuring the
Cs is essential. To accomplish this, measuring the  Cs/
Cs/ Cs isotope ratio can be useful, so this study optimized a solvent extraction method, with calix[4]arene-bis(t-octylbenzo-crown-6) [BOBCalixC6] in 1-octanol, to purify radioactive Cs, radiocesium, from a solution of major environmental soil elements and mass spectrometry interference elements. This optimized method was applied to Cs purification in soil samples (40 g), and the final solutions contained a total of 10
Cs isotope ratio can be useful, so this study optimized a solvent extraction method, with calix[4]arene-bis(t-octylbenzo-crown-6) [BOBCalixC6] in 1-octanol, to purify radioactive Cs, radiocesium, from a solution of major environmental soil elements and mass spectrometry interference elements. This optimized method was applied to Cs purification in soil samples (40 g), and the final solutions contained a total of 10 g/ml of the major soil elements and ng/ml concentrations at most of interfering elements. Soil samples collected near the FDNPS were then purified, and the
g/ml of the major soil elements and ng/ml concentrations at most of interfering elements. Soil samples collected near the FDNPS were then purified, and the  Cs/
Cs/ Cs isotope ratios were measured, using both thermal ionization mass spectrometry (TIMS) and triple quadrupole induced coupled plasma mass spectrometry (ICP-QQQ). The results of each of these measurements were compared, and we found that Cs isotope ratios obtained by TIMS were more precise, by an order of magnitude, while the ICP-QQQ results possessed good abundance sensitivities. A slightly higher
Cs isotope ratios were measured, using both thermal ionization mass spectrometry (TIMS) and triple quadrupole induced coupled plasma mass spectrometry (ICP-QQQ). The results of each of these measurements were compared, and we found that Cs isotope ratios obtained by TIMS were more precise, by an order of magnitude, while the ICP-QQQ results possessed good abundance sensitivities. A slightly higher  Cs/
Cs/ Cs ratio in the northwest area of the FDNPS was observed, while other areas exhibited similar values, all within the measurement error range, which indicated different origins of radiocesium. These results agreed with previously reported
Cs ratio in the northwest area of the FDNPS was observed, while other areas exhibited similar values, all within the measurement error range, which indicated different origins of radiocesium. These results agreed with previously reported  Cs/
Cs/ Cs activity distributions, suggesting that this ratio may be useful in identifying radiocesium origins for evaluating future nuclear reactor decommissions.
Cs activity distributions, suggesting that this ratio may be useful in identifying radiocesium origins for evaluating future nuclear reactor decommissions.
Sawaguchi, Takuma
"Yugai Haikibutsu, Hoshasei Haikibutsu Eno Semento, Konkurito Gijutsu No Tekiyo Kenkyu Iinkai" Hokokusho (CD-ROM), p.165 - 173, 2020/12
no abstracts in English
Yamaguchi, Tetsuji; Ohira, Saki; Hemmi, Ko; Barr, L.; Shimada, Asako; Maeda, Toshikatsu; Iida, Yoshihisa
Radiochimica Acta, 108(11), p.873 - 877, 2020/11
Times Cited Count:7 Percentile:66.68(Chemistry, Inorganic & Nuclear)Iida, Yoshihisa; Nakadoi, Yasumasa; Yamaguchi, Tetsuji
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 24(1), p.53 - 64, 2017/06
The information on the contaminated water treatment in Fukushima Daiichi Nuclear Power Station announced by Tokyo Electric Power Co., Inc. (TEPCO) were summarized in terms of the management of the secondary wastes, for the purpose of accumulating technical knowledge for long-term storage of the wastes. Concerns for the long-term soundness of waste storage containers were pointed out as follows, corrosion of stainless steel containers exposed to radiation in the presence of chloride ions, corrosion of stainless steel containers under acidic conditions or in the presence of activated carbon, and radiation degradation of the high-performance container (HIC) in which slurry was stored.
 activity and temperature on the dissolution rate of compacted montmorillonite under highly alkaline conditions [Clay Minerals, vol.51, p.275 (2016), Corrected Fig. 7.]
activity and temperature on the dissolution rate of compacted montmorillonite under highly alkaline conditions [Clay Minerals, vol.51, p.275 (2016), Corrected Fig. 7.]Sawaguchi, Takuma; Tsukada, Manabu; Yamaguchi, Tetsuji; Mukai, Masayuki
Clay Minerals, 51(5), P. 815, 2016/12
ERRATUM; Effects of OH activity and temperature on the dissolution rate of compacted montmorillonite under highly alkaline conditions [Clay Minerals, vol.51, p.275 (2016), Corrected Fig. 7.]
activity and temperature on the dissolution rate of compacted montmorillonite under highly alkaline conditions [Clay Minerals, vol.51, p.275 (2016), Corrected Fig. 7.]
 activity and temperature on the dissolution rate of compacted montmorillonite under highly alkaline conditions
activity and temperature on the dissolution rate of compacted montmorillonite under highly alkaline conditionsSawaguchi, Takuma; Tsukada, Manabu; Yamaguchi, Tetsuji; Mukai, Masayuki
Clay Minerals, 51(2), p.267 - 278, 2016/05
Times Cited Count:7 Percentile:24.47(Chemistry, Physical)The dependences of the dissolution rate of compacted montmorillonite on activity of OH (a
(a -) and temperature (T) were investigated. The dissolution rate of montmorillonite (
-) and temperature (T) were investigated. The dissolution rate of montmorillonite (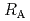 ) in compacted pure montmorillonite, which was formulized as
) in compacted pure montmorillonite, which was formulized as 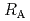 = 10
= 10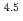 (a
(a -)
-)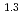 e
e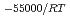 , was higher than that in the compacted sand-bentonite mixtures:
, was higher than that in the compacted sand-bentonite mixtures: 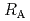 = 3500 (a
= 3500 (a -)
-)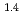 e
e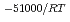 . The difference can be explained by considering the decrease in a
. The difference can be explained by considering the decrease in a - in the mixtures accompanied by dissolution of accessory minerals such as quartz and chalcedony. The dissolution rate model developed for pure montmorillonite is expected to be applied to bentonite mixtures if quantification of the decrease in a
- in the mixtures accompanied by dissolution of accessory minerals such as quartz and chalcedony. The dissolution rate model developed for pure montmorillonite is expected to be applied to bentonite mixtures if quantification of the decrease in a - is achieved somehow.
- is achieved somehow.
Yamaguchi, Tetsuji; Sawaguchi, Takuma; Tsukada, Manabu; Hoshino, Seiichi*; Tanaka, Tadao
Clay Minerals, 51(2), p.279 - 287, 2016/02
Times Cited Count:7 Percentile:24.47(Chemistry, Physical)Alteration of bentonite-cement interfaces and accompanying changes in diffusivity of tritiated water was experimentally investigated using intact hardened cement specimens. The alteration by carbonate solution was accompanied by mineralogical changes at the interface and a decrease in the diffusivity to 70% of the initial value after 180-day period. Another alteration under silicate system contacting hardened cement and compacted bentonite was accompanied by mineralogical changes at the interface and a decrease in the diffusivity to 71% of the initial value after 600-day period. The changes in the diffusivity were much less than those observed for mixed specimens of granulated hardened cement and bentonite where the diffusivity decreased down to 20% of the initial value over 180 days. The results were extrapolated to 15 years under simple assumptions and showed good agreement with those observed in the cement-argillite interface at Tournemire URL. Such an explanation enhances our confidence in our assessment of alteration of cement-bentonite systems and can be a base for using our data and models in long term assessment of radioactive waste disposal.
Yamaguchi, Tetsuji; Shimada, Taro; Ishibashi, Makoto*; Akagi, Yosuke*; Kurosawa, Mitsuru*; Matsubara, Akiyoshi*; Matsuda, Yuki*; Sato, Shigeyoshi*
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 22(2), p.21 - 27, 2015/12
It is predictable from previous studies that radiocesium hardly migrate into surrounding soils and groundwater from soils contaminated by the Fukushima Daiichi Nuclear Power Plant accident if they are buried and covered with indigenous soils. This study demonstrated the prediction by performing in-situ migration experiments over a year in a public park in Miho, Ibaraki prefecture and in two public parks in Misato, Saitama prefecture. Contaminated soils were buried at a depth range of 0.3 - 1.0 m or at 0.3 - 1.3 m and covered with indigenous soil layer of 0.3 m, and were sprinkled with water to accelerate the radiocesium migration. Migration of radiocesium was not observed from radiometric analyses of boring cores and soil water samples. Laboratory column and sorption experiments revealed that the radiocesium hardly leach out of the soil and even if they leach out from the contaminated soil, radiocesium is sorbed on surrounding soils and hardly migrate through the soli layer. Simulation of Cs-137 migration for 100 years by an advection-diffusion model showed that Cs-137 hardly migrate and decay out in the contaminated soil.
Kudo, Tamotsu; Onizawa, Kunio*; Nakamura, Takehiko
JAEA-Evaluation 2015-011, 209 Pages, 2015/11
Japan Atomic Energy Agency (JAEA) consulted an assessment committee, "Evaluation Committee of Research and Development (R&D) Activities for Nuclear Safety", for post- and pre-review assessment of R&D on nuclear safety research. In response to JAEA's request, the Committee assessed mainly the progress of the R&D project according to guidelines, which addressed the rationale behind the R&D project, the relevance of the project outcome and the efficiency of the project implementation during the period of the current and next plan. As a result, the Committee concluded that the progress of the R&D project is satisfactory. This report describes the results of evaluation by the Committee. In addition, the appendix of this report contains presentations used for the evaluation, and responses from JAEA on the comments from the member of the Committee.
Tanaka, Tadao
Proceedings of 23rd International Conference on Nuclear Engineering (ICONE-23) (DVD-ROM), 5 Pages, 2015/05
It is necessary to confirm that there is no significant radioactivity remaining on NPP site, for the site release beforehand. Cesium 137 ( Cs) is the typical radionuclide caused from NPP. In this research, generation of particulate
Cs) is the typical radionuclide caused from NPP. In this research, generation of particulate  Cs species at ground surface and its migration behavior were examined. Migration experiments were carried out by a column method, in which deionized water was fed intermittently at the drying interval for 7 days into a sand layer contaminated with
Cs species at ground surface and its migration behavior were examined. Migration experiments were carried out by a column method, in which deionized water was fed intermittently at the drying interval for 7 days into a sand layer contaminated with  Cs. A portion of the
Cs. A portion of the  Cs in the upper surface region, which formed particulate species by sorbing on fine particles, migrated into the deeper layer. Fine particle itself also was generated at the sand surface by weathering. The sand was weathered during the drying period, so that small amount of fine particles including
Cs in the upper surface region, which formed particulate species by sorbing on fine particles, migrated into the deeper layer. Fine particle itself also was generated at the sand surface by weathering. The sand was weathered during the drying period, so that small amount of fine particles including  Cs was newly dissociated from the sand. Such particulate
Cs was newly dissociated from the sand. Such particulate  Cs species may be accumulated slowly by repeated cycles of rainfall and drying, during long term.
Cs species may be accumulated slowly by repeated cycles of rainfall and drying, during long term.
Maeda, Toshikatsu; Watanabe, Koichi; Omori, Hiroyuki*; Sakamaki, Keiko; Inagaki, Yaohiro*; Idemitsu, Kazuya*
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 21(2), p.63 - 74, 2014/12
Static leach tests were conducted for simulated HLW glass in CaCl /Ca(OH)
/Ca(OH) solutions to investigate the corrosion behavior of HLW glass under calcium-rich environments induced by cement based materials in geological repositories. Another series of leach tests were conducted in deionized water in the presence of iron to investigate the effects of iron over-pack on the glass corrosion. In Ca solutions, corrosion of the glass was inhibited during the test period compared to that in deionized water, while the corrosion was enhanced at the presence of iron. The enhancement of the glass corrosion was assumed to be accompanied with transformation of silica, a glass network former, into iron silicates.
solutions to investigate the corrosion behavior of HLW glass under calcium-rich environments induced by cement based materials in geological repositories. Another series of leach tests were conducted in deionized water in the presence of iron to investigate the effects of iron over-pack on the glass corrosion. In Ca solutions, corrosion of the glass was inhibited during the test period compared to that in deionized water, while the corrosion was enhanced at the presence of iron. The enhancement of the glass corrosion was assumed to be accompanied with transformation of silica, a glass network former, into iron silicates.
Yamaguchi, Tetsuji; Takeda, Seiji; Nishimura, Yuki; Iida, Yoshihisa; Tanaka, Tadao
Radiochimica Acta, 102(11), p.999 - 1008, 2014/11
Times Cited Count:1 Percentile:8.88(Chemistry, Inorganic & Nuclear)An attempt was made to select thermodynamic data with uncertainties and to evaluate the solubility of radioelements with uncertainties considering variation in groundwater chemistry. The thermodynamic data was selected by reviewing the JAEA-TDB released in 2012. Data for Nb, Pd and Pa were revised from the viewpoint of the consistency of the data selection process. Data for Se, U and Pa were revised from the viewpoint of the conservativeness. Up-to-date ternary calcium-metal(IV)-OH complexes were adopted for Zr, Th, U, Np and Pu. A Monte Carlo-based probabilistic calculation code, PA-SOL, was used for probabilistic analysis of the solubility.
Sakamaki, Keiko; Kataoka, Masaharu; Maeda, Toshikatsu; Iida, Yoshihisa; Kamoshida, Michio; Yamaguchi, Tetsuji; Tanaka, Tadao
Corrosion Engineering, Science and Technology, 49(6), p.450 - 454, 2014/09
Times Cited Count:0 Percentile:0.01(Materials Science, Multidisciplinary)Corrosion experiments of a carbon steel plate embedded in bentonite mixture were conducted toverify our models assessing Eh evolution induced by corrosion of carbon steel overpack. Theexperimental results showed that the Eh decreased for the first 200 days and was subsequentlystabilised at around -450 mV; corrosion products were identified as magnetite and Fe waspresent mostly as divalent Fe within a 5 mm range from the carbon steel plate. Reactive transportmodelling was performed to assess the Eh evolution in the system using kinetic dissolution modelfor metallic iron and thermodynamic equilibrium models for other chemical reactions and closelyreproduced the experimental results. The models were verified only under the conditionsemployed in this study.
Tanaka, Tadao; Shimada, Taro; Sukegawa, Takenori
Progress in Nuclear Science and Technology (Internet), 4, p.832 - 835, 2014/04
According to a basic policy of Japan, nuclear power plant sites are allowed to be released from nuclear safety regulations after the plants are decommissioned. It is necessary to confirm that there is no significant radioactivity remaining on the sites, for the site release beforehand. Cobalt 60 is one of the typical radionuclide for nuclear power plants. In the evaluation concept, all of cobalt 60, which is in reality distributed across the area of interest, are assumed to be the single point source located at the furthest position on the surface of the area from a Ge detector. In such a configuration, minimum detectable time was supplied by Monte Carlo calculations, and the minimum detectable time was approximately equal to the actual measurement time of the point source by the Ge detector. These results mean that the proposed evaluation method was reasonable for the conservative evaluation of cobalt 60 remaining in the nuclear power plant sites.
Sawaguchi, Takuma; Kadowaki, Mitsushi*; Yamaguchi, Tetsuji; Mukai, Masayuki; Tanaka, Tadao
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 20(2), p.71 - 78, 2013/12
The dissolution rate for montmorillonite under compacted condition was studied in order to evaluate long-term alteration behavior of bentonite buffer materials by highly alkaline groundwater. The dissolution rate of compacted montmorillonite was found to be larger than that of montmorillonite in compacted sand-bentonite mixtures at 130  C, which revealed that the dissolution of montmorillonite was inhibited by decreasing the activity of hydroxide ions (
C, which revealed that the dissolution of montmorillonite was inhibited by decreasing the activity of hydroxide ions (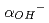 ) in the compacted mixtures including accessory minerals such as silica. In order to provide reliability for the analysis of bentonite-alteration using a formula of dissolution rate of montmorillonite, it is important to quantify the decrease of
) in the compacted mixtures including accessory minerals such as silica. In order to provide reliability for the analysis of bentonite-alteration using a formula of dissolution rate of montmorillonite, it is important to quantify the decrease of 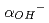 in the compacted mixtures and to formulate the dissolution rate of compacted montmorillonite.
in the compacted mixtures and to formulate the dissolution rate of compacted montmorillonite.
Sakamaki, Keiko
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 20(2), p.109 - 110, 2013/12
no abstracts in English
Yamaguchi, Tetsuji; Sawaguchi, Takuma; Tsukada, Manabu; Kadowaki, Mitsushi*; Tanaka, Tadao
Clay Minerals, 48(2), p.403 - 410, 2013/05
Times Cited Count:8 Percentile:23.68(Chemistry, Physical)Montmorillonite is the main constituent of bentonite clay buffer materials in radioactive waste repositories. Highly alkaline environments induced by cement based materials are likely to alter montmorillonite, and to deteriorate the physical and/or chemical properties of the buffer materials. The deterioration may cause variation in hydraulic conductivity of the buffer and induce major uncertainties in the radionuclide migration analysis. Empirical data on the variation of hydraulic conductivity are, however, scarce mainly because the alteration of compacted buffer materials, sand-bentonite mixture specimen, is extremely slow. In this study, laboratory experiments were performed to observe changes in hydraulic conductivity of sand-bentonite mixtures accompanied with their alkaline alteration using NaOH based solutions at 80-90  C. Three types of experiments proved that the alkaline alteration of bentonite buffer can increase the hydraulic conductivity. The data obtained in this study are useful for verification of the code that will be used for assessing the alteration.
C. Three types of experiments proved that the alkaline alteration of bentonite buffer can increase the hydraulic conductivity. The data obtained in this study are useful for verification of the code that will be used for assessing the alteration.
Yamashita, Yuji*; Tanaka, Tadao; Adachi, Yasuhisa*
Colloids and Surfaces A; Physicochemical and Engineering Aspects, 417, p.230 - 235, 2013/01
Times Cited Count:11 Percentile:28.04(Chemistry, Physical)Transport behavior of humic acid was studied from the aspects of colloidal stability. Deposition kinetics of purified Aldrich humic acid was investigated over a wide range of monovalent and divalent electrolyte concentrations at pH 3.0 by using packed-bed technique. Spherical glass beads with diameters of 0.2 mm were utilized as model collectors. Breakthrough curves of humic acid were measured with UV-VIS spectrophotometer. The values of C/C0 (C and C0 are the effluent and influent concentrations of humic acid, respectively) decreased with increasing electrolyte concentrations. Experimental collision efficiencies were determined from measured single collector efficiencies. The results are presented as stability curves, which is the logarithm of the collision efficiency as a function of the logarithm of salt concentration. Stability curves of humic acid obviously showed favorable and unfavorable regimes and critical deposition concentration in the presence of both electrolytes. As a consequence, it is indicated that transport behavior of humic acid is primarily controlled by electrostatic interaction between humic acids and collectors.