Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Katsuoka, Nanako; Akiyama, Daisuke*; Kirishima, Akira*; Nagai, Takayuki; Okamoto, Yoshihiro; Sato, Nobuaki*
2023-Nendo "Busshitsu, Debaisu Ryoiki Kyodo Kenkyu Kyoten" Kenkyu Seika, Katsudo Hokokusho (Internet), 1 Pages, 2024/07
no abstracts in English
 CoSi
CoSiYamauchi, Hiroki; Sari, D. P.*; Yasui, Yukio*; Sakakura, Terutoshi*; Kimura, Hiroyuki*; Nakao, Akiko*; Ohara, Takashi; Honda, Takashi*; Kodama, Katsuaki; Igawa, Naoki; et al.
Physical Review Research (Internet), 6(1), p.013144_1 - 013144_9, 2024/02
Nagai, Takayuki; Katsuoka, Nanako; Okamoto, Yoshihiro; Baba, Yuji*; Akiyama, Daisuke*
Photon Factory Activity Report 2023 (Internet), 3 Pages, 2024/00
no abstracts in English
Nagai, Takayuki; Akiyama, Daisuke*; Kirishima, Akira*; Katsuoka, Nanako; Okamoto, Yoshihiro; Sato, Nobuaki*
2022-Nendo "Busshitsu, Debaisu Ryoiki Kyodo Kenkyu Kyoten" Kenkyu Seika, Katsudo Hokokusho (Internet), 1 Pages, 2023/09
no abstracts in English
Nagai, Takayuki; Tone, Masaya; Katsuoka, Nanako; Okamoto, Yoshihiro; Baba, Yuji*; Akiyama, Daisuke*
Photon Factory Activity Report 2022 (Internet), 3 Pages, 2023/00
no abstracts in English
Nagai, Takayuki; Okamoto, Yoshihiro; Baba, Yuji*; Akiyama, Daisuke*; Arima, Tatsumi*
Photon Factory Activity Report 2021 (Internet), 2 Pages, 2022/00
no abstracts in English
Nagai, Takayuki; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Okamoto, Yoshihiro
2020-Nendo "Busshitsu, Debaisu Ryoiki Kyodo Kenkyu Kyoten" Oyobi "Hito, Kankyo To Busshitsu O Tsunagu Inobeshion Soshutsu Dainamikku, Araiansu" Kenkyu Seika, Katsudo Hokokusho (CD-ROM), 1 Pages, 2021/11
no abstracts in English
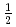 anisotropic triangular antiferromagnet Ca
anisotropic triangular antiferromagnet Ca ReO
ReO Cl
Cl
Nawa, Kazuhiro*; Hirai, Daigoro*; Kofu, Maiko; Nakajima, Kenji; Murasaki, Ryo*; Kogane, Satoshi*; Kimata, Motoi*; Nojiri, Hiroyuki*; Hiroi, Zenji*; Sato, Taku*
Physical Review Research (Internet), 2(4), p.043121_1 - 043121_11, 2020/12
The spin excitations of the 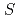 =
= 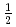 anisotropic triangular antiferromagnet Ca
anisotropic triangular antiferromagnet Ca ReO
ReO Cl
Cl were investigated by inelastic neutron-scattering experiments. The spin excitation spectrum exhibits sharp dispersive modes in addition to a spinonlike continuum. The consistency with the simulated spectrum based on the random-phase approximation is better for Ca
were investigated by inelastic neutron-scattering experiments. The spin excitation spectrum exhibits sharp dispersive modes in addition to a spinonlike continuum. The consistency with the simulated spectrum based on the random-phase approximation is better for Ca ReO
ReO Cl
Cl than for Cs
than for Cs CuCl
CuCl , indicating that the spin system in the former remains closer to a Tomonaga-Luttinger liquidlike disordered state.
, indicating that the spin system in the former remains closer to a Tomonaga-Luttinger liquidlike disordered state.
Nagai, Takayuki; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Okamoto, Yoshihiro
2019-Nendo "Busshitsu, Debaisu Ryoiki Kyodo Kenkyu Kyoten" Oyobi "Hito, Kankyo To Busshitsu O Tsunagu Inobeshion Soshutsu Dainamikku, Araiansu" Kenkyu Seika, Katsudo Hokokusho (CD-ROM), P. 20191107_1, 2020/11
no abstracts in English
Nagai, Takayuki; Okamoto, Yoshihiro; Akiyama, Daisuke*; Uehara, Akihiro*; Fujii, Toshiyuki*; Sekimoto, Shun*
KURNS Progress Report 2019, P. 257, 2020/08
To understand the influence of glass structural change by neutron irradiation and boron isotope composition, glass samples were made from enrichment boric acid reagents and neutron irradiation of those samples was carried out in Pn-2 of KUR. The structural change of glass sample after the irradiation will be estimated in 2020FY. Before neutron irradiation test of glass samples, the Si-O bridging structure difference by boron isotope composition compared by using a Raman spectrometry.
Nagai, Takayuki; Shimoyama, Iwao; Okamoto, Yoshihiro; Akiyama, Daisuke*; Arima, Tatsumi*
Photon Factory Activity Report 2019 (Internet), 3 Pages, 2020/00
no abstracts in English
Nagai, Takayuki; Kobayashi, Hidekazu; Okamoto, Yoshihiro; Akiyama, Daisuke*; Sato, Nobuaki*; Uehara, Akihiro*; Fujii, Toshiyuki*; Sekimoto, Shun*
KURNS Progress Report 2018, P. 105, 2019/08
To understand this structural change of a borosilicate glass by a neutron irradiation in detail, the irradiation test was carried out in KUR in 2017FY. The glass structure was estimated by using Raman spectrometry in 2018FY. Comparing with the Raman spectra of glass samples before and after irradiation, it could be observed the change of peak height of Si-O bridging structure by the irradiation.
Nagai, Takayuki; Akiyama, Daisuke*; Sato, Nobuaki*; Okamoto, Yoshihiro
"Busshitsu, Debaisu Ryoiki Kyodo Kenkyu Kyoten" Oyobi "Hito, Kankyo To Busshitsu O Tsunagu Inobeshion Soshutsu Danamikku, Araiansu" Kenkyu Seika, Katsudo Hokokusho (Heisei-30-Nendo) (CD-ROM), P. 20181080_1, 2019/06
no abstracts in English
Nagai, Takayuki; Okamoto, Yoshihiro; Akiyama, Daisuke*; Sato, Nobuaki*
Photon Factory Activity Report 2018 (Internet), 2 Pages, 2019/00
no abstracts in English
Nagai, Takayuki; Akiyama, Daisuke*; Sato, Nobuaki*
Busshitsu, Debaisu Ryoiki Kyodo Kenkyu Kyoten Kenkyu Seika Hokokusho (Heisei-29-Nendo) (CD-ROM), 1 Pages, 2018/04
no abstracts in English
Nagai, Takayuki; Kobayashi, Hidekazu; Okamoto, Yoshihiro; Akiyama, Daisuke*; Sato, Nobuaki*
Photon Factory Activity Report 2017, 2 Pages, 2018/00
no abstracts in English
Nagai, Takayuki; Akiyama, Daisuke*; Sato, Nobuaki*; Sasage, Kenichi
Busshitsu, Debaisu Ryoiki Kyodo Kenkyu Kyoten Kenkyu Seika Hokokusho (Heisei-28-Nendo) (CD-ROM), 1 Pages, 2017/03
no abstracts in English
Nagai, Takayuki; Sato, Nobuaki*; Sasage, Kenichi
Busshitsu, Debaisu Ryoiki Kyodo Kenkyu Kyoten Kenkyu Seika Hokokusho (Heisei-27-Nendo) (CD-ROM), 2 Pages, 2016/03
no abstracts in English
 /UO
/UO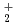 couple in Li
couple in Li MoO
MoO -Na
-Na MoO
MoO eutectic melt at 550
eutectic melt at 550 C
CNagai, Takayuki; Uehara, Akihiro*; Fujii, Toshiyuki*; Sato, Nobuaki*; Kofuji, Hirohide; Myochin, Munetaka; Yamana, Hajimu*
Journal of Nuclear Materials, 454(1-3), p.159 - 163, 2014/11
Times Cited Count:1 Percentile:8.02(Materials Science, Multidisciplinary)The redox equilibrium of UO /UO
/UO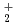 couple was measured in Li
couple was measured in Li MoO
MoO -Na
-Na MoO
MoO eutectic melt at 550
eutectic melt at 550 C by cyclic voltammetry and absorption spectrophotometry. The standard redox potential of UO
C by cyclic voltammetry and absorption spectrophotometry. The standard redox potential of UO /UO
/UO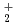 couple was approximately evaluated by cyclic voltammetry. Further, the absorption spectrum and equilibrium potential were measured, repeatedly adding UO
couple was approximately evaluated by cyclic voltammetry. Further, the absorption spectrum and equilibrium potential were measured, repeatedly adding UO source material into the melt containing UO
source material into the melt containing UO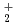 . From the correlation between the equilibrium potential of the melt and the concentration ratio [UO
. From the correlation between the equilibrium potential of the melt and the concentration ratio [UO ]/[UO
]/[UO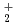 ] spectrophotometrically evaluated, the standard redox potential of UO
] spectrophotometrically evaluated, the standard redox potential of UO /UO
/UO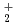 couple was determined to be -0.847
couple was determined to be -0.847 0.010 V vs. O
0.010 V vs. O /O
/O .
.
 C
CNagai, Takayuki; Uehara, Akihiro*; Fukushima, Mineo; Myochin, Munetaka; Fujii, Toshiyuki*; Sato, Nobuaki*; Yamana, Hajimu*
Proceedings in Radiochemistry, 1(1), p.151 - 155, 2011/09
Absorption spectra of dissolved uranium species in molten Li MoO
MoO -Na
-Na MoO
MoO eutectic at 550
eutectic at 550  C were measured by spectrophotometry, and their redox reactions were also investigated by cyclic voltammetry. Observed absorption spectra of uranium species were similar to those of UO
C were measured by spectrophotometry, and their redox reactions were also investigated by cyclic voltammetry. Observed absorption spectra of uranium species were similar to those of UO
 in molten chlorides. After purging oxygen into the melt, the absorption peaks of UO
in molten chlorides. After purging oxygen into the melt, the absorption peaks of UO
 decreased and UO
decreased and UO
 was thought to be oxidized to UO
was thought to be oxidized to UO
 . When the uranium species were not contained in the melt, we confirmed that alkali metals deposited at -0.7 V and a small reduction of this melt was observed at -0.3 V. When UO
. When the uranium species were not contained in the melt, we confirmed that alkali metals deposited at -0.7 V and a small reduction of this melt was observed at -0.3 V. When UO was dissolved into the melt, the reduction of the uranium species was observed at -0.2 V. It was suggested that the dissolved uranium species are recovered as mixed uranium-molybdenum oxides by electrolysis.
was dissolved into the melt, the reduction of the uranium species was observed at -0.2 V. It was suggested that the dissolved uranium species are recovered as mixed uranium-molybdenum oxides by electrolysis.