Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ueda, Yuki; Micheau, C.; Akutsu, Kazuhiro*; Tokunaga, Kohei; Yamada, Masako*; Yamada, Norifumi*; Bourgeois, D.*; Motokawa, Ryuhei
Langmuir, 40(46), p.24257 - 24271, 2024/11
Times Cited Count:0 Percentile:0.00(Chemistry, Multidisciplinary)Microscopic structures in liquid-liquid extraction, such as structuration between extractants or extracted complexes in bulk organic phases and at interfaces, can influence macroscopic phenomena, such as the distribution behavior of solutes, including extraction efficiency, selectivity, and phase separation of the organic phase. In this study, we correlated the macroscopic behavior of the extraction of Zr(IV) ions from nitric acid solutions with microscopic structural information in order to understand at the molecular level the key factors contributing to the higher metal ion extraction performance in the fluorous extraction system comprising fluorous phosphate (TFP) in perfluorohexane as compared to the analogous organic extraction system comprising organic phosphate (THP) in n-hexane. Extended X-ray absorption fine structure, neutron reflectometry, and small-angle neutron scattering revealed the local coordination structure around the Zr(IV) ion, the accumulation of extractant molecules at the interface, and the structuration of extractant molecules in the bulk extraction phase, respectively. In the fluorous extraction system, extractant aggregates with were formed after contact with nitric acid. In contrast, in the organic extraction system, only extractant dimers were formed. We speculate that differences in the local coordination structure around the Zr(IV) ion and the structuration of the extractant molecules in the bulk extraction phase contribute to the high Zr(IV) extraction performance in the fluorous extraction system. In particular, the size of the aggregates hardly changed with increasing Zr(IV) concentration in the fluorous phase, which may be closely related to the absence of phase splitting in the fluorous extraction system.
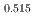 Gd
Gd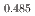 Mn
Mn O
O
Ishii, Yuta*; Sakakura, Terutoshi*; Ishikawa, Yoshihisa*; Kiyanagi, Ryoji; Lustikova, J.*; Aoyama, Takuya*; Ogushi, Kenya*; Wakabayashi, Yusuke*; Kimura, Hiroyuki*; Noda, Yukio*
Physical Review B, 110(18), p.184404_1 - 184404_7, 2024/11
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)Matsuura, Masato*; Zhang, J.*; Kamimura, Yasushi*; Kofu, Maiko; Edagawa, Keiichi*
Physical Review Letters, 133(13), p.136101_1 - 136101_5, 2024/09
Times Cited Count:1 Percentile:48.32(Physics, Multidisciplinary)Micheau, C.; Ueda, Yuki; Motokawa, Ryuhei; Akutsu, Kazuhiro*; Yamada, Norifumi*; Yamada, Masako*; Moussaoui, S. A.*; Makombe, E.*; Meyer, D.*; Berthon, L.*; et al.
Journal of Molecular Liquids, 401, p.124372_1 - 124372_12, 2024/05
Times Cited Count:2 Percentile:65.57(Chemistry, Physical) complex exhibiting intermolecular proton shifting coupled spin transition
complex exhibiting intermolecular proton shifting coupled spin transitionJi, T.*; Su, S.*; Wu, S.*; Hori, Yuta*; Shigeta, Yasuteru*; Huang, Y.*; Zheng, W.*; Xu, W.*; Zhang, X.*; Kiyanagi, Ryoji; et al.
Angewandte Chemie; International Edition, 63(25), p.e202404843_1 - e202404843_6, 2024/04
Times Cited Count:0 Percentile:0.00(Chemistry, Multidisciplinary)Higuchi, Yuki*; Yoshimune, Wataru*; Kato, Satoru*; Hibi, Shogo*; Setoyama, Daigo*; Isegawa, Kazuhisa*; Matsumoto, Yoshihiro*; Hayashida, Hirotoshi*; Nozaki, Hiroshi*; Harada, Masashi*; et al.
Communications Engineering (Internet), 3, p.33_1 - 33_7, 2024/02
Taniguchi, Koji*; Huang, P.-J.*; Sagayama, Hajime*; Kiyanagi, Ryoji; Oishi, Kazuki*; Kito, Shunsuke*; Nakamura, Yuiga*; Miyasaka, Hitoshi*
Physical Review Materials (Internet), 8(2), p.024409_1 - 024409_10, 2024/02
Times Cited Count:2 Percentile:56.91(Materials Science, Multidisciplinary) CoSi
CoSiYamauchi, Hiroki; Sari, D. P.*; Yasui, Yukio*; Sakakura, Terutoshi*; Kimura, Hiroyuki*; Nakao, Akiko*; Ohara, Takashi; Honda, Takashi*; Kodama, Katsuaki; Igawa, Naoki; et al.
Physical Review Research (Internet), 6(1), p.013144_1 - 013144_9, 2024/02
Kofu, Maiko; Kawamura, Seiko; Murai, Naoki; Ishii, Rieko*; Hirai, Daigoro*; Arima, Hiroshi*; Funakoshi, Kenichi*
Physical Review Research (Internet), 6(1), p.013006_1 - 013006_9, 2024/01
Zhang, A.*; Deng, K.*; Sheng, J.*; Liu, P.*; Kumar, S.*; Shimada, Kenya*; Jiang, Z.*; Liu, Z.*; Shen, D.*; Li, J.*; et al.
Chinese Physics Letters, 40(12), p.126101_1 - 126101_8, 2023/12
Times Cited Count:11 Percentile:83.08(Physics, Multidisciplinary)Nozaki, Hiroshi*; Kondo, Hiroki*; Shinohara, Takenao; Setoyama, Daigo*; Matsumoto, Yoshihiro*; Sasaki, Tsuyoshi*; Isegawa, Kazuhisa*; Hayashida, Hirotoshi*
Scientific Reports (Internet), 13, p.22082_1 - 22082_8, 2023/12
Times Cited Count:3 Percentile:24.30(Multidisciplinary Sciences)Shibata, Motoki*; Nakanishi, Yohei*; Abe, Jun*; Arima, Hiroshi*; Iwase, Hiroki*; Shibayama, Mitsuhiro*; Motokawa, Ryuhei; Kumada, Takayuki; Takata, Shinichi; Yamamoto, Katsuhiro*; et al.
Polymer Journal, 55(11), p.1165 - 1170, 2023/11
Times Cited Count:2 Percentile:19.24(Polymer Science)Nakanishi, Takumi*; Hori, Yuta*; Shigeta, Yasuteru*; Sato, Hiroyasu*; Kiyanagi, Ryoji; Munakata, Koji*; Ohara, Takashi; Okazawa, Atsushi*; Shimada, Rintaro*; Sakamoto, Akira*; et al.
Journal of the American Chemical Society, 145(35), p.19177 - 19181, 2023/08
Times Cited Count:5 Percentile:51.73(Chemistry, Multidisciplinary)Takagi, Hirotaka*; Takagi, Rina*; Minami, Susumu*; Nomoto, Takuya*; Oishi, Kazuki*; Suzuki, Michito*; Yanagi, Yuki*; Hirayama, Motoaki*; Khanh, N.*; Karube, Kosuke*; et al.
Nature Physics, 19(7), p.961 - 968, 2023/07
Times Cited Count:50 Percentile:99.07(Physics, Multidisciplinary)

 Sn
Sn (
( = Rh and Ir)
= Rh and Ir)Shimoda, Ami*; Iwasa, Kazuaki*; Kuwahara, Keitaro*; Sagayama, Hajime*; Nakao, Hironori*; Ishikado, Motoyuki*; Ohara, Takashi; Nakao, Akiko*; Hoshikawa, Akinori*; Ishigaki, Toru*
JPS Conference Proceedings (Internet), 38, p.011091_1 - 011091_6, 2023/05
Micheau, C.; Ueda, Yuki; Akutsu, Kazuhiro*; Bourgeois, D.*; Motokawa, Ryuhei
Solvent Extraction and Ion Exchange, 41(2), p.221 - 240, 2023/02
Times Cited Count:4 Percentile:43.72(Chemistry, Multidisciplinary)Tamura, Kazuhisa; Akutsu-Suyama, Kazuhiro*; Cagnes, M.*; Darwish, T. A.*
ECS Advances (Internet), 1(2), p.020503_1 - 020503_5, 2022/06
The ionic liquid/Si electrode interface was investigated using neutron reflectivity. We precisely elucidated the structure of the electrical double layer formed at 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)amide ([BMIM]TFSA)/Si(100) electrode interface with the orientation of the [BMIM]TFSA molecule using a partially deuterated [BMIM]TFSA. The results revealed that [BMIM]TFSA molecules form a layered structure. Cation and anion molecules are alternatingly stacked and molecules in the first three layers are horizontally oriented to the electrode surface at E = -1.2 V, i.e., on the negatively charged electrode surface. It was also revealed that the imidazole ring in [BMIM] cation is parallel to the electrode surface.
Yamashita, Keishiro*; Komatsu, Kazuki*; Ohara, Takashi; Munakata, Koji*; Irifune, Tetsuo*; Shimmei, Toru*; Sugiyama, Kazumasa*; Kawamata, Toru*; Kagi, Hiroyuki*
High Pressure Research, 42(1), p.121 - 135, 2022/03
Times Cited Count:4 Percentile:47.58(Physics, Multidisciplinary)Hashimoto, Shunsuke*; Nakajima, Kenji; Kikuchi, Tatsuya*; Kamazawa, Kazuya*; Shibata, Kaoru; Yamada, Takeshi*
Journal of Molecular Liquids, 342, p.117580_1 - 117580_8, 2021/11
Times Cited Count:4 Percentile:24.04(Chemistry, Physical)Quasi-elastic neutron scattering (QENS) and pulsed-field-gradient nuclear magnetic resonance (PFGNMR) analyses of a nanofluid composed of silicon dioxide (SiO ) nanoparticles and a base fluid of ethylene glycol aqueous solution were performed. The aim was to elucidate the mechanism increase in the thermal conductivity of the nanofluid above its theoretical value. The obtained experimental results indicate that SiO
) nanoparticles and a base fluid of ethylene glycol aqueous solution were performed. The aim was to elucidate the mechanism increase in the thermal conductivity of the nanofluid above its theoretical value. The obtained experimental results indicate that SiO particles may decrease the self-diffusion coefficient of the liquid molecules in the ethylene glycol aqueous solution because of their highly restricted motion around these nanoparticles. At a constant temperature, the thermal conductivity increases as the self-diffusion coefficient of the liquid molecules decreases in the SiO
particles may decrease the self-diffusion coefficient of the liquid molecules in the ethylene glycol aqueous solution because of their highly restricted motion around these nanoparticles. At a constant temperature, the thermal conductivity increases as the self-diffusion coefficient of the liquid molecules decreases in the SiO nanofluids.
nanofluids.
Kofu, Maiko; Watanuki, Ryuta*; Sakakibara, Toshiro*; Kawamura, Seiko; Nakajima, Kenji; Matsuura, Masato*; Ueki, Takeshi*; Akutsu, Kazuhiro*; Yamamuro, Osamu*
Scientific Reports (Internet), 11(1), p.12098_1 - 12098_8, 2021/06
Times Cited Count:8 Percentile:58.52(Multidisciplinary Sciences)