Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Miyashita, Sunao; Oe, Kazuhiro; Toyoshima, Atsushi; Sato, Tetsuya; Asai, Masato; Tsukada, Kazuaki; Nagame, Yuichiro; Sch del, M.; Kaneya, Yusuke*; Omtvedt, J. P.*; et al.
del, M.; Kaneya, Yusuke*; Omtvedt, J. P.*; et al.
no journal, ,
For the experiments of the redox potentials of Sg, rapid separation between different oxidation states of Sg is needed. In this study, solvent extraction of hexavalent Mo and W, as the lighter homologues of Sg, from 1.0 mol/L hydrochloric acid solution into di-(2-ethylhexyl)phosphoric acid (HDEHP), 1-phenyl-3-metyl-4-benzoyl-5-pyrazolone (PMBP), N-benzoyl-N-pheny-hydroxylamine (BPHA) and 4-isopropyl-tropolone (HT) in toluene was carried out. When HDEHP and PMBP were used as extractant, extraction equilibrium of  W was achieved within 5 and 1 hours respectively. On the other hand, when BPHA and HT were used, extraction equilibrium of
W was achieved within 5 and 1 hours respectively. On the other hand, when BPHA and HT were used, extraction equilibrium of  W was quickly achieved within 1 minute in both extractants. Extraction kinetics of
W was quickly achieved within 1 minute in both extractants. Extraction kinetics of 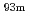 Mo using BPHA and HT was examined. Extraction kinetics of Mo was slower than that of W. Extraction equilibrium of Mo was achieved within 10 by BPHA and 3 minutes by HT. We concluded that HT is a suitable extractant for the Sg reduction experiment.
Mo using BPHA and HT was examined. Extraction kinetics of Mo was slower than that of W. Extraction equilibrium of Mo was achieved within 10 by BPHA and 3 minutes by HT. We concluded that HT is a suitable extractant for the Sg reduction experiment.
Toyoshima, Atsushi; Miyashita, Sunao*; Asai, Masato; Sato, Tetsuya; Kaneya, Yusuke; Tsukada, Kazuaki; Kitatsuji, Yoshihiro; Nagame, Yuichiro; Sch del, M.; Lerum, H. V.*; et al.
del, M.; Lerum, H. V.*; et al.
no journal, ,
To carry out a continuous reduction experiment of Sg with the low production rates and the short half-life, we are developing a new chemistry assembly consisting of a membrane degasser (MDG), a flow electrolytic column (FEC), the continuous liquid-liquid extraction apparatus, and the liquid scintillation counting system (SISAK). Recently, we have begun preparatory studies with Mo and W isotopes. Aqueous solution dissolving Mo and W was successfully separated from a carrier gas. Redox couples of Mo(VI)/Mo(V) and W(VI)/W(V) in HCl have been characterized for their macro amounts. Extraction behavior of Mo(VI) and W(VI) between toluene containing hinokitiol (HT) and HCl was successfully observed by a batch method. On-line extractions of short-lived Mo and W were also carried out using SISAK and MDG. In the symposium, our present status of the preparation with Mo and W will be presented.
Toyoshima, Atsushi; Asai, Masato; Attallah, M. F.*; Goto, Naoya*; Gupta, N. S.*; Haba, Hiromitsu*; Huang, M.*; Kanaya, Jumpei*; Kaneya, Yusuke; Kasamatsu, Yoshitaka*; et al.
no journal, ,
Towards electrolytic reduction of Sg, batch-wise electrolytic reduction of carrier-free 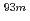 Mo and
Mo and 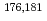 W radiotracers was studied using a flow electrolytic column (FEC). The electrolyzed samples from a FEC were chemically analyzed by solvent extraction with TOA and HDEHP to separate and identify reduced species from the stable Mo(VI) and W(VI) ones based on their different extraction behavior.
W radiotracers was studied using a flow electrolytic column (FEC). The electrolyzed samples from a FEC were chemically analyzed by solvent extraction with TOA and HDEHP to separate and identify reduced species from the stable Mo(VI) and W(VI) ones based on their different extraction behavior. 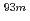 Mo and
Mo and 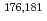 W were applied as radiotracers. We also performed cyclic voltammetry and UV/Vis absorption spectrometry of macro amounts of Mo and W in acidic solutions to obtain information on redox reactions of these elements under given conditions. In the conference, the present status of the preparatory reduction experiments with Mo and W will be presented.
W were applied as radiotracers. We also performed cyclic voltammetry and UV/Vis absorption spectrometry of macro amounts of Mo and W in acidic solutions to obtain information on redox reactions of these elements under given conditions. In the conference, the present status of the preparatory reduction experiments with Mo and W will be presented.
Toyoshima, Atsushi; Miyashita, Sunao*; Oe, Kazuhiro*; Kitayama, Yuta*; Lerum, H. V.*; Goto, Naoya*; Kaneya, Yusuke; Komori, Yukiko*; Mitsukai, Akina*; Vascon, A.; et al.
no journal, ,
no abstracts in English
Toyoshima, Atsushi; Oe, Kazuhiro*; Asai, Masato; Attallah, M. F.*; Goto, Naoya*; Gupta, N. S.*; Haba, Hiromitsu*; Kaneko, Masashi*; Kaneya, Yusuke; Kasamatsu, Yoshitaka*; et al.
no journal, ,
Due to short half-lives less than 10 s and extremely low production rates, transactinide elements heavier than seaborgium (Sg) are produced on an atom per hour scale. Therefore, a continuous rapid chemistry assembly is required to study aqueous-phase chemistry of these heaviest elements. In the present study, we started developments of a continuous chemistry assembly. Our first attempt was made in on-line experiments with Mo and W, lighter homologs of Sg, to optimize a chemistry assembly consisting of a newly developed membrane degasser as an interface between gas-jet and aqueous phase, a flow electrolytic column apparatus utilized to control oxidation states of Mo and W ions, and the continuous liquid-liquid extraction apparatus of SISAK for separation. In the conference, present status of the developments will be presented.