Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yamazaki, Yuji*; Tamada, Taro; Kasai, Noriyuki*; Urakawa, Itaru*; Aono, Yukiko*; Hasegewa, Hisashi*; Fujita, Toshiro*; Kuroki, Ryota; Yamashita, Takeyoshi*; Fukumoto, Seiji*; et al.
Journal of Bone and Mineral Research, 23(9), p.1509 - 1518, 2008/09
Times Cited Count:150 Percentile:94.53Fibroblast growth factor (FGF)23 is proposed to play a physiological role in the regulation of phosphate and vitamin D metabolism; deranged circulatory levels of FGF23 cause several diseases with abnormal mineral metabolism. We developed two antibodies (FN1 and FC1) that recognize the N- and C-terminal regions of FGF23, respectively. Both FN1 and FC1 inhibited FGF23 activity in a cell-based Klotho-dependent reporter assay. The present study using neutralizing antibodies confirms that FGF23 is a physiological regulator of phosphate and vitamin D metabolism. Then, we addressed the mechanism of action for these neutralizing antibodies. Structural analysis of the FGF23/FN1-Fab complex revealed that FN1 masked putative FGF receptor-binding sites in the N-terminal domain of FGF23, while biochemical analyses showed that FC1 interfered with the association between FGF23 and Klotho by binding to the C-terminal domain of FGF23. Taken together, our results suggest that the N- and C-terminal domains of FGF23 are responsible for association with cognate FGF receptors and Klotho, respectively, and that these interactions are indispensable for FGF23 activity.
Arai, Shigeki; Tamada, Taro; Honjo, Eijiro; Maeda, Yoshitake*; Kuroki, Ryota
no journal, ,
The mouse antibody TN1 recognizes the human thrombopoietin (hTPO) that primarily stimulates megakaryocytopoiesis and platelet production. By the structural comparison between TN1-Fab itselt (hTPO unbound form) and hTPO bound form of TN1-Fab (PDB id 1V7M and 1V7N), we found that the CDR of TN1-Fab need not accompany the large conformational changes of upon TPO recognition. Moreover, the isothermal titration calorimetry showed that the conformational entropy change upon the hTPO binding to the TN1-Fab was  446.4 kJ/mol/K, corresponding to 2,920
446.4 kJ/mol/K, corresponding to 2,920 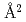 burying upon hTPO binding. This change of accessible surface area is larger than that of the previous result (1,580
burying upon hTPO binding. This change of accessible surface area is larger than that of the previous result (1,580 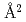 ) estimated from the crystal structure of hTPO / TN1-Fab complex. Since the CDR structure of TN1-Fab did not change, the change in surface area may be from the conformational change of hTPO upon the binding to Fab.
) estimated from the crystal structure of hTPO / TN1-Fab complex. Since the CDR structure of TN1-Fab did not change, the change in surface area may be from the conformational change of hTPO upon the binding to Fab.
Arai, Shigeki; Tamada, Taro; Maeda, Yoshitake*; Kuroki, Ryota
no journal, ,
The mouse antibody TN1 recognizes the human thrombopoietin (hTPO) that primarily stimulates megakaryocytopoiesis and platelet production. In order to clarify the mechanism of the neutralizing activity of the TN1 antibody, the crystal structure of TN1-Fab was determined to 2.0 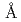 resolution and was compared with that of TN1-Fab / hTPO complex (PDB id 1V7M and 1V7N). The relative angle of the variable- and constant-regions of the hTPO unbound form of TN1-Fab was slightly shifted from those of TN1-Fab / hTPO complex (rms deviation = 2.4
resolution and was compared with that of TN1-Fab / hTPO complex (PDB id 1V7M and 1V7N). The relative angle of the variable- and constant-regions of the hTPO unbound form of TN1-Fab was slightly shifted from those of TN1-Fab / hTPO complex (rms deviation = 2.4 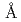 for all C
for all C atoms of Fab). On the other hand, only the slight shift of the side chain of CDR was observed (rmsd
atoms of Fab). On the other hand, only the slight shift of the side chain of CDR was observed (rmsd  1.5
1.5 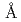 for atoms of side chain of each CDR) upon recognition of the epitope of hTPO. This structural shift of side chain of paratope did not accompany the
for atoms of side chain of each CDR) upon recognition of the epitope of hTPO. This structural shift of side chain of paratope did not accompany the 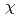 -angle rotation, but the parallel shift. These results indicate that the CDR of TN1-Fab need not accompany the large conformational changes of upon TPO recognition.
-angle rotation, but the parallel shift. These results indicate that the CDR of TN1-Fab need not accompany the large conformational changes of upon TPO recognition.
Tamada, Taro; Honjo, Eijiro; Maeda, Yoshitake*; Okamoto, Tomoyuki*; Ishibashi, Matsujiro*; Tokunaga, Masao*; Kuroki, Ryota
no journal, ,
We have succeeded in crystallization of 2:2 complex between human granulocyte colony-stimulating factor (hGCSF) and the Ig-like and CRH domains of human GCSF-R (hGCSF-R) and determined its tertiary structure by X-ray crystallography at 2.8  resolution. The signaling 2:2 complex is formed via cross-over interactions between the Ig-like domain of hGCSF-R and the neighboring hGCSF, forming a two-fold axis of crystallographic symmetry. This conformation is quite different from that of the heterogeneous mGCSF-R complex, and more closely resembles the 2:2:2 active assembly of human interleukin-6 (IL-6), human IL-6
resolution. The signaling 2:2 complex is formed via cross-over interactions between the Ig-like domain of hGCSF-R and the neighboring hGCSF, forming a two-fold axis of crystallographic symmetry. This conformation is quite different from that of the heterogeneous mGCSF-R complex, and more closely resembles the 2:2:2 active assembly of human interleukin-6 (IL-6), human IL-6  -receptor and human gp130 (which is a shared signal transducing receptor for several cytokines), and the 2:2 assembly of viral IL-6 and human gp130. The Ig-like domain cross-over structure necessary for GCSF-R activation is consistent with previously reported thermodynamic and mutational analyses.
-receptor and human gp130 (which is a shared signal transducing receptor for several cytokines), and the 2:2 assembly of viral IL-6 and human gp130. The Ig-like domain cross-over structure necessary for GCSF-R activation is consistent with previously reported thermodynamic and mutational analyses.
Kuroki, Ryota
no journal, ,
We have succeeded in preparation and crystallization of 2:2 complex between human GCSF (hGCSF) and the Ig-like and CRH domains of human GCSF-R (hGCSF-R) and determined its tertiary structure by X-ray crystallography at 2.8 angstrom resolution. The signaling 2:2 complex is formed via cross-over interactions between the Ig-like domain of hGCSF-R and the neighboring hGCSF, forming a two-fold axis of crystallographic symmetry. This conformation is quite different from that of the heterogeneous mGCSF-R complex, and more closely resembles the 2:2:2 active assembly of human interleukin-6 (IL-6), human IL-6-receptor and human gp130 (which is a shared signal transducing receptor for several cytokines), and the 2:2 assembly of viral IL-6 and human gp130. The Ig-like domain cross-over structure necessary for GCSF-R activation is consistent with previously reported thermodynamic and mutational analyses.
Tamada, Taro; Honjo, Eijiro; Maeda, Yoshitake*; Kuroki, Ryota
no journal, ,
Granulocyte colony-stimulating factor (GCSF) has become an important cytokine for medical treatment of patients suffering from granulopoenia. Here, we report the crystal structure of a complex between human GCSF (hGCSF) and the Ig-like and CRH domains of human GCSF-R (hGCSF-R) at 2.8 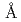 resolution. The signaling 2:2 complex is formed via cross-over interactions between the Ig-like domain of hGCSF-R and the neighboring hGCSF, forming a two-fold axis of crystallographic symmetry. This conformation is quite different from that of the heterogeneous mGCSF-R complex, and more closely resembles the 2:2:2 active assembly of human interleukin-6 (IL-6), human IL-6 alpha-receptor and human gp130 (which is a shared signal transducing receptor for several cytokines), and the 2:2 assembly of viral IL-6 and human gp130. The Ig-like domain cross-over structure necessary for GCSF-R activation is consistent with previously reported thermodynamic and mutational analyses.
resolution. The signaling 2:2 complex is formed via cross-over interactions between the Ig-like domain of hGCSF-R and the neighboring hGCSF, forming a two-fold axis of crystallographic symmetry. This conformation is quite different from that of the heterogeneous mGCSF-R complex, and more closely resembles the 2:2:2 active assembly of human interleukin-6 (IL-6), human IL-6 alpha-receptor and human gp130 (which is a shared signal transducing receptor for several cytokines), and the 2:2 assembly of viral IL-6 and human gp130. The Ig-like domain cross-over structure necessary for GCSF-R activation is consistent with previously reported thermodynamic and mutational analyses.
Tamada, Taro; Honjo, Eijiro; Arai, Shigeki; Kuroki, Ryota
no journal, ,
The ligand-receptor interaction in the extracellular environment is essential for signal transduction of biological processes. We have focused on the structure/function relationships of these proteins. Granulocyte colony-stimulating factor (GCSF) has become an important cytokine for medical treatment of patients suffering from granulopoenia through regulating the maturation, proliferation, and differentiation of the precursor cells of neutrophilic granulocytes. We determined a crystal structure of the signaling complex between human GCSF and a ligand binding region of GCSF receptor (GCSF-R). We also determined additional structures of human cytokines and extracellular regions of cytokine receptors, where the complexation with an antibody fragment (Fab) was used for crystallization and structure determination.