Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...

Kawaguchi, Kazuhiro*; Yamamoto, Shunya; Yoshikawa, Masahito; Takahiro, Katsumi*
Thin Solid Films, 562, p.648 - 652, 2014/07
Times Cited Count:4 Percentile:19.55(Materials Science, Multidisciplinary)Liquid organic-hydrides such as cyclohexane which can release-absorbing hydrogen in reversible have been proposed approvable carriers to store and transport hydrogen. However, organic-hydrides are known as highly flammable in air. To realize practical use of this hydrogen production process, the monitoring of leakage of organic-hydride gas is strongly required. In the present work, we have examined the plasmonic sensing ability of Au nanoparticle (NP) arrays for dilute cyclohexane. Au NP arrays were prepared on SiO by a sputter deposition technique. The change in an extinction spectrum of Au NP arrays before and after exposure of cyclohexane vapor enabled us to detect it. The Au NP array prepared with 4.4
by a sputter deposition technique. The change in an extinction spectrum of Au NP arrays before and after exposure of cyclohexane vapor enabled us to detect it. The Au NP array prepared with 4.4  10
10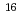 Au atoms/cm
Au atoms/cm deposited at 300
deposited at 300 C was and demonstrated a high sensitivity for cyclohexane at RT. The experimentally detectable concentration of cyclohexane was as low as 0.5 vol%, much lower than its explosion limit (1.3 vol%).
C was and demonstrated a high sensitivity for cyclohexane at RT. The experimentally detectable concentration of cyclohexane was as low as 0.5 vol%, much lower than its explosion limit (1.3 vol%).
 /Si by ion implantation
/Si by ion implantationTakahiro, Katsumi*; Ninakuchi, Yuki*; Kawaguchi, Kazuhiro; Isshiki, Toshiyuki*; Nishio, Koji*; Sasase, Masato*; Yamamoto, Shunya; Nishiyama, Fumitaka*
Applied Surface Science, 258(19), p.7322 - 7326, 2012/07
Times Cited Count:2 Percentile:10.02(Chemistry, Physical)A nanometer-sized metallic particle embedded in a transparent dielectric exhibits a nonlinear susceptibility, and going to be applied to nonlinear optical devices. In the present study, well-ordered arrangements of Ag nanoparticles have been found for Ag-implanted SiO . Thermally grown SiO
. Thermally grown SiO on Si were implanted with 350 keV-Ag ions to fluences of 0.37-1.2
on Si were implanted with 350 keV-Ag ions to fluences of 0.37-1.2  10
10 ions/cm
ions/cm . Cross-sectional transmission electron microscopy and scanning transmission electron microscopy reveal the presence of a two-dimensional array of Ag nanoparticles of 25-40 nm in diameter located at a depth of
. Cross-sectional transmission electron microscopy and scanning transmission electron microscopy reveal the presence of a two-dimensional array of Ag nanoparticles of 25-40 nm in diameter located at a depth of  130 nm, together with the self-organization of tiny Ag nanoparticles aligned along the SiO
130 nm, together with the self-organization of tiny Ag nanoparticles aligned along the SiO /Si interface. X-ray photoelectron spectroscopy and X-ray diffraction confirm the stability of these Ag nanoparticles embedded in the SiO
/Si interface. X-ray photoelectron spectroscopy and X-ray diffraction confirm the stability of these Ag nanoparticles embedded in the SiO /Si is found to be stable against oxidation and sulfidation when stored in ambient conditions for more than one and a half year.
/Si is found to be stable against oxidation and sulfidation when stored in ambient conditions for more than one and a half year.
Kitatsuji, Yoshihiro; Kimura, Takaumi; Kihara, Sorin*
Electrochimica Acta, 74, p.215 - 221, 2012/07
Times Cited Count:2 Percentile:5.39(Electrochemistry)Redox reactions of U, Np and Pu ions of various oxidation states were investigated by flow electrolysis at a column electrode (CE) equipped with a platinized glassy carbon (GC) fiber working electrode (Pt/GC-WE), and compared with those observed at the CE with an ordinary activated GC fiber working electrode (GC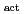 -WE). Since the overpotential for the reduction of NpO
-WE). Since the overpotential for the reduction of NpO
 and PuO
and PuO
 were decreased when Pt/GC-WE was employed, the one-electron reduction of NpO
were decreased when Pt/GC-WE was employed, the one-electron reduction of NpO
 to Np
to Np followed by that of Np
followed by that of Np to Np
to Np and the one-step three-electron reduction of PuO
and the one-step three-electron reduction of PuO
 to Pu
to Pu proceeded at the CE with Pt/GC-WE, different from the reduction processes of NpO
proceeded at the CE with Pt/GC-WE, different from the reduction processes of NpO
 and PuO
and PuO
 at the CE with GC
at the CE with GC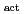 -WE. A rapid and precise method for the preparation of U, Np and Pu ion of a desired oxidation state was proposed by taking advantage of the unique characteristics of the CE with Pt/GC-WE.
-WE. A rapid and precise method for the preparation of U, Np and Pu ion of a desired oxidation state was proposed by taking advantage of the unique characteristics of the CE with Pt/GC-WE.
Kawaguchi, Kazuhiro; Saito, Masahiro*; Takahiro, Katsumi*; Yamamoto, Shunya; Yoshikawa, Masahito
Plasmonics, 6(3), p.535 - 539, 2011/09
Times Cited Count:8 Percentile:28.2(Chemistry, Physical)Silver nanoparticles (Ag NPs) have optical absorption bands due to localized surface plasmon resonance (LSPR) in visible range. The LSPR of Ag-NPs is being used for optical gas sensors. However, there have been differences in LSPR absorption band between most of the experimental data and calculations. We have demonstrated that plasma treatments for Ag NPs to clear the effect of contamination on surface of Ag NPs for LSPR absorption band. The results show that Ar plasma treatments to Ag NPs bring blue-shift and narrowing in their LSPR absorption band. Raman scattering analysis result that hydrocarbons adsorbed on silver surfaces were removed effectively by plasma exposure. It was found that the decrease in Raman line intensity for hydrocarbons was correlated well with the blue-shift. Our findings indicate that one of the most important factors for difference in LSPR absorption band between the experimental data and calculations is due to the impurity adsorption on silver surfaces.
Kitatsuji, Yoshihiro; Okugaki, Tomohiko*; Kasuno, Megumi*; Kubota, Hiroki*; Maeda, Koji*; Kimura, Takaumi; Yoshida, Zenko; Kihara, Sorin*
Journal of Chemical Thermodynamics, 43(6), p.844 - 851, 2011/06
Times Cited Count:8 Percentile:30.28(Thermodynamics)Standard Gibbs energies for transfer ( G
G
 ) of actinyl ions (AnO
) of actinyl ions (AnO
 ; z = 2 or 1; An: U, Np or Pu) between an aqueous solution and an organic solution were determined based on distribution method combined with voltammetry for ion transfer at the interface of two immiscible electrolyte solutions. The organic solutions examined were nitrobenzene, 1,2-dichloroethane, benzonitrile, acetophenone and 2-nitrophenyl octyl ether. Irrespective of the type of organic solutions,
; z = 2 or 1; An: U, Np or Pu) between an aqueous solution and an organic solution were determined based on distribution method combined with voltammetry for ion transfer at the interface of two immiscible electrolyte solutions. The organic solutions examined were nitrobenzene, 1,2-dichloroethane, benzonitrile, acetophenone and 2-nitrophenyl octyl ether. Irrespective of the type of organic solutions,  G
G
 of UO
of UO
 , NpO
, NpO
 and PuO
and PuO
 were nearly equal to each other and slightly larger than that of Mg
were nearly equal to each other and slightly larger than that of Mg . The
. The  G
G
 of NpO
of NpO
 was extraordinary large compared with those of ordinary monovalent cations. The dependence of
was extraordinary large compared with those of ordinary monovalent cations. The dependence of  G
G
 of AnO
of AnO
 on the type of organic solutions was similar to that of H
on the type of organic solutions was similar to that of H or Mg
or Mg . The
. The  G
G
 of An
of An and An
and An were also discussed briefly.
were also discussed briefly.
Kitatsuji, Yoshihiro; Kimura, Takaumi; Kihara, Sorin*
Journal of Electroanalytical Chemistry, 641(1-2), p.83 - 89, 2010/03
Unique current-time relations were observed during the controlled potential electrolysis for the reduction of Np(V) to Np(IV) or Np(III) at a Au or Pt electrode. The reduction process of Np(V) was elucidated based on bulk electrolysis and voltammetry: Though Np(V) is not reduced directly at a Au electrode, it is reduced by chemical reaction with Np(III) which is produced by the electrode reduction of Np(IV). The reduction of Np(V) at a Pt electrode is initiated by the electrocatalytic reduction to Np(IV) with the hydrogen atom which is produced by the electrode reduction of H and adsorbed on the Pt electrode surface. The Np(IV) produced functions as an electron transfer mediator to reduce Np(V) similarly to the reaction at a Au electrode. The above described catalytic reduction processes of Np(V) were elucidated with the aid of digital simulation. Methods useful for the preparation of Np(IV) and Np(III) by bulk electrolysis were proposed based on the experimental results.
and adsorbed on the Pt electrode surface. The Np(IV) produced functions as an electron transfer mediator to reduce Np(V) similarly to the reaction at a Au electrode. The above described catalytic reduction processes of Np(V) were elucidated with the aid of digital simulation. Methods useful for the preparation of Np(IV) and Np(III) by bulk electrolysis were proposed based on the experimental results.

Takahiro, Katsumi*; Oizumi, Shinnosuke*; Morimoto, Keiichi*; Kawatsura, Kiyoshi*; Isshiki, Toshiyuki*; Nishio, Koji*; Nagata, Shinji*; Yamamoto, Shunya; Narumi, Kazumasa; Naramoto, Hiroshi*
Applied Surface Science, 256(4), p.1061 - 1064, 2009/11
Times Cited Count:8 Percentile:36.66(Chemistry, Physical)Takahiro, Katsumi*; Ozaki, Koichi*; Kawatsura, Kiyoshi*; Nagata, Shinji*; Yamamoto, Shunya; Narumi, Kazumasa; Naramoto, Hiroshi*
Applied Surface Science, 256(4), p.972 - 975, 2009/11
Times Cited Count:12 Percentile:47.84(Chemistry, Physical)Okugaki, Tomohiko*; Kitatsuji, Yoshihiro; Kasuno, Megumi*; Yoshizumi, Asuka*; Kubota, Hiroki*; Shibafuji, Yayoi*; Maeda, Koji*; Yoshida, Zenko; Kihara, Sorin*
Journal of Electroanalytical Chemistry, 629(1-2), p.50 - 56, 2009/04
A high performance electrochemical solvent extraction method was developed based on the quantitative transfer of ions from an aqueous, W, to an organic solution, O. It was realized by applying a potential difference between W and O. The cell was composed of a porous Teflon tube immersed with O, a Ag wire inserted into the tube, a Pt wire placed outside the tube. When W containing ions was forced to flow through the narrow gap between the tube and Ag wire, and potential was applied, a very rapid quantitative ion transfer was attained. When O containing extractant, more than 99% of U(VI) in W was extracted during the residence (e.g., 40 s) in the cell. The fundamental feature of the extraction system was investigated, taking into account the application of the system to the extraction of actinide, lanthanide, Sr or Cs ions. The use of a column electrode system connected before the extraction system was examined in order to adjust the oxidation state of the element to that desired.
Furusawa, Toshiharu*; Suzuki, Eiko*; Nagaoka, Shunji*; Suzuki, Hiromi*; Ishioka, Noriaki*; Hamada, Nobuyuki*; Wada, Seiichi*; Kobayashi, Yasuhiko; Sakashita, Tetsuya; Kakizaki, Takehiko*; et al.
JAEA-Review 2007-060, JAEA Takasaki Annual Report 2006, P. 115, 2008/03
Using heavy ion microbeam, we investigated the somatic mutation arising on the larvae in embyro and yolk in the egg of silkworm,  . The incidence of the somatic mutation was 12%, and the same level of mutation following the microbeam irradiation at the center of the egg. However, the microbeam irradiation to the abdomen of the silkworm larvae induced the increase of somatic mutation, 63% (3 Gy) and 80% (6 Gy).
. The incidence of the somatic mutation was 12%, and the same level of mutation following the microbeam irradiation at the center of the egg. However, the microbeam irradiation to the abdomen of the silkworm larvae induced the increase of somatic mutation, 63% (3 Gy) and 80% (6 Gy).
 organic solution interface in chelate extraction
organic solution interface in chelate extractionUehara, Akihiro*; Kasuno, Megumi*; Okugaki, Tomohiko*; Kitatsuji, Yoshihiro; Shirai, Osamu*; Yoshida, Zenko; Kihara, Sorin*
Journal of Electroanalytical Chemistry, 604(2), p.115 - 124, 2007/06
The distribution ratio of a metal ion between an aqueous solution and an organic solution in solvent extraction with a chelating agent was evaluated by using physicochemical constants determined electrochemically such as standard Gibbs energies for transfers of the ions, the overall complex formation constants and the acid dissociation constants of the chelating agent. The distribution ratio thus evaluated agreed well with those determined by the distribution experiment.
 , egg by the incidence of somatic mutation appearing in the larvae
, egg by the incidence of somatic mutation appearing in the larvaeFurusawa, Toshiharu*; Suzuki, Eiko*; Nagaoka, Shunji*; Suzuki, Hiromi*; Ishioka, Noriaki*; Hamada, Nobuyuki*; Wada, Seiichi*; Kobayashi, Yasuhiko; Sakashita, Tetsuya; Kakizaki, Takehiko; et al.
JAEA-Review 2006-042, JAEA Takasaki Annual Report 2005, P. 118, 2007/02
no abstracts in English
Takahiro, Katsumi*; Oizumi, Shinnosuke*; Terai, Atsushi*; Kawatsura, Kiyoshi*; Tsuchiya, Bun*; Nagata, Shinji*; Yamamoto, Shunya; Naramoto, Hiroshi; Narumi, Kazumasa; Sasase, Masato*
Journal of Applied Physics, 100(8), p.084325_1 - 084325_6, 2006/10
Times Cited Count:28 Percentile:68.36(Physics, Applied)X-ray photoelectron spectroscopy (XPS) has been applied for size estimation of Au clusters formed by ion implantation into glassy carbon. The 4f and 5d XPS spectra reveal the presence of the cluster 0.7-2.5nm in diameter, depending on the Au concentration. The elationship between XPS 4f-binding energy shift and 5d splitting is determined for the Au clusters embedded in the carbon and found to be significantly different from the previous data for the ones supported on a carbon substrate. We suppose that this difference results from the effect of the environment around a cluster on Coulomb charging during photoemission at the final state.
Takahiro, Katsumi*; Terai, Atsushi*; Kawatsura, Kiyoshi*; Naramoto, Hiroshi; Yamamoto, Shunya; Tsuchiya, Bun*; Nagata, Shinji*; Nishiyama, Fumitaka*
Japanese Journal of Applied Physics, Part 1, 45(3A), p.1823 - 1825, 2006/03
Times Cited Count:2 Percentile:8.72(Physics, Applied)It is found that the surface charging during Rutherford backscattering spectrometry (RBS) of insulating sapphire samples enables us to measure the charge-state distribution of probing ions backscattered at the sapphire surface. For Cu/Au-deposited Al O
O samples, two components, higher and lower-energy ones, were resolved on both Cu and Au peaks in the RBS random spectrum. For single-crystalline Al
samples, two components, higher and lower-energy ones, were resolved on both Cu and Au peaks in the RBS random spectrum. For single-crystalline Al O
O samples, a double-peak structure was clearly observed on both Al and O surface peaks in the RBS aligned spectrum. The charge-state distribution can be obtained from the intensity of each component. The results obtained here are compared with previous data for the equilibrium charge-state distribution.
samples, a double-peak structure was clearly observed on both Al and O surface peaks in the RBS aligned spectrum. The charge-state distribution can be obtained from the intensity of each component. The results obtained here are compared with previous data for the equilibrium charge-state distribution.
Takahiro, Katsumi*; Terai, Atsushi*; Oizumi, Shinnosuke*; Kawatsura, Kiyoshi*; Yamamoto, Shunya; Naramoto, Hiroshi
Nuclear Instruments and Methods in Physics Research B, 242(1-2), p.445 - 447, 2006/01
Times Cited Count:9 Percentile:53.56(Instruments & Instrumentation)X-ray photoelectron spectroscopy (XPS) and Raman spectroscopy have been applied to investigate amorphization processes by ion irradiation for various carbon materials, including highly oriented pyrolytic graphite (HOPG), isotropic graphite, glassy carbon (GC) and C crystalline film. It is found that the asymmetry of the XPS C 1s line increases as the irradiation dose increases. The origin of the asymmetry appeared on the C 1s line is discussed. It is concluded that the asymmetry of the C 1s line is not correlated with the increase in the size of a graphitic layer, but is related with the structural disorders, such as a bond angle disorder.
crystalline film. It is found that the asymmetry of the XPS C 1s line increases as the irradiation dose increases. The origin of the asymmetry appeared on the C 1s line is discussed. It is concluded that the asymmetry of the C 1s line is not correlated with the increase in the size of a graphitic layer, but is related with the structural disorders, such as a bond angle disorder.
Yoshizumi, Asuka*; Uehara, Akihiro*; Kasuno, Megumi*; Kitatsuji, Yoshihiro; Yoshida, Zenko; Kihara, Sorin*
Journal of Electroanalytical Chemistry, 581(2), p.275 - 283, 2005/08
no abstracts in English
Aoyagi, Hisao*; Kitatsuji, Yoshihiro; Yoshida, Zenko; Kihara, Sorin*
Analytica Chimica Acta, 538(1-2), p.283 - 289, 2005/05
Times Cited Count:15 Percentile:41.62(Chemistry, Analytical)Redox behavior of Np(III), (IV), (V) and (VI) ions in aqueous perchloric, nitric and sulphuric acid solutions was elucidated by using column electrodes connected in series in a flow system. Using a glassy carbon fiber working electrode as the column electrode was found to be very useful for the investigation of current-potential relations of not only reversible redox processes such as Np(VI)/(V) or Np(IV)/(III) but also irreversible processes such as Np(V)/(IV) or Np(V)/(III), the latter not observed by conventional voltammetry which uses a glassy carbon or platinum electrode. Quantitative electrolysis could be executed even if the redox process was completely irreversible by the use of the column electrodes. By taking advantage of column electrode electrolysis, a novel method was developed for the rapid preparation of a neptunium species of a desired oxidation state, including unstable species. The multi-step column electrode system was demonstrated to be useful for the coulometric determination and speciation of Np(IV), (V) and (VI) in aqueous HNO solution as an example.
solution as an example.
Yoshida, Zenko; Kihara, Sorin*; Fujinaga, Taichiro*
Bunseki Kagaku, 53(4), p.195 - 205, 2004/04
Times Cited Count:1 Percentile:1.01(Chemistry, Analytical)A quantitative electrolysis of an electro-active species in a sample solution can be achieved very rapidly when the solution is allowed to flow through and be electrolyzed at a column electrode. Complete electrolysis of the species is attained with a small over-voltage, even if the electrode reaction is slow. The electrolytic method using the column electrode is suitable for automated or remote-controlled operation. Because of unmatched advantages, the column-electrode electrolysis method has been widely applied to the coulometric determination of species in a flowing sample solution and to liquid chromatography as a coulometric detector. This technique is especially favorable for elucidating mechanism of such complicated reactions as that involving unstable intermediates. In the present article, a principle and a feature of the column-electrode electrolysis are presented and the advantages of these methods are reviewed based on recent works on its application to flow-injection analysis and to a study of the redox reaction of actinide ions or the reaction of short-lived radicals.
 organic solution interface
organic solution interfaceUehara, Akihiro*; Yoshida, Zenko; Yoshida, Yumi*; Kitatsuji, Yoshihiro; Kasuno, Megumi*; Maeda, Koji*; Kihara, Sorin*
Journal of Electroanalytical Chemistry, 563(2), p.257 - 267, 2004/03
no abstracts in English
Imai, Makoto*; Sataka, Masao; Kawatsura, Kiyoshi*; Takahiro, Katsumi*; Komaki, Kenichiro*; Shibata, Hiromi*; Sugai, Hiroyuki; Nishio, Katsuhisa
no journal, ,
no abstracts in English