Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 -alkylated 2-pyrrolidone derivatives
-alkylated 2-pyrrolidone derivativesTakao, Koichiro*; Kawata, Yoshihisa*; Nogami, Masanobu*; Harada, Masayuki*; Morita, Yasuji; Nishimura, Kenji*; Ikeda, Yasuhisa*
Journal of Nuclear Science and Technology, 52(2), p.294 - 298, 2015/02
Times Cited Count:2 Percentile:17.57(Nuclear Science & Technology)Yields of precipitated UO (NO
(NO )
) (NRP)
(NRP) (NRP =
(NRP =  -alkylated 2-pyrrolidone) were precisely determined by considering reduction of the solution volume through the precipitation, which can be estimated from difference in acid concentrations of the liquid phases before and after the precipitation. The studied NRPs were
-alkylated 2-pyrrolidone) were precisely determined by considering reduction of the solution volume through the precipitation, which can be estimated from difference in acid concentrations of the liquid phases before and after the precipitation. The studied NRPs were  -
- -butyl (NBP) and
-butyl (NBP) and  -
- -propyl (NProP) derivatives. In both systems, the precipitation yields precisely determined were always higher than those simply calculated from the ratio of uranium concentrations before and after the precipitation. However, the differences between them are in the range of 0.6% - 2.6%. If such a difference is practically negligible, the volume reduction through the precipitation does not have to be taken into account for simplicity of the analytical manipulation.
-propyl (NProP) derivatives. In both systems, the precipitation yields precisely determined were always higher than those simply calculated from the ratio of uranium concentrations before and after the precipitation. However, the differences between them are in the range of 0.6% - 2.6%. If such a difference is practically negligible, the volume reduction through the precipitation does not have to be taken into account for simplicity of the analytical manipulation.
Nogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Journal of Radioanalytical and Nuclear Chemistry, 283(2), p.541 - 546, 2010/02
Times Cited Count:20 Percentile:78.77(Chemistry, Analytical)Adsorptivity of polyvinylpolypyrrolidone (PVPP) to various metal ions was examined as a part of the development of resins with selectivity to U(VI) in nitric acid media. It was found that PVPP has a strong adsorptivity to U(VI) in wide concentration range of nitric acid. The mechanism of U(VI) adsorption by PVPP was discussed by results of Scatchard plot analysis and infrared spectroscopic analysis. Furthermore, it was found that fission productions except for Re(VII) as the simulant of Tc(VII) and Pd(II) are not adsorbed on to PVPP and that Pd(II) and Re(VII) species are weakly adsorbed in the lower concentration ranges of nitric acid, where the adsorption rates of Pd(II) are extremely slower than those of U(VI). These results indicate that U(VI) can be separated from other metal ions by PVPP.
 -ray irradiation
-ray irradiationNogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Kawata, Yoshihisa; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
JAEA-Review 2009-041, JAEA Takasaki Annual Report 2008, P. 25, 2009/12
As a part of the development of a novel reprocessing system for spent FBR fuels based on the precipitation method, influence of concentrations of HNO on the stability by
on the stability by  -ray irradiation was examined for
-ray irradiation was examined for  -
- -butyl-2-pyrrolidone (NBP), a candidate precipitant for the first precipitation step for selectively precipitating U(VI). The residual ratios of the samples for HNO
-butyl-2-pyrrolidone (NBP), a candidate precipitant for the first precipitation step for selectively precipitating U(VI). The residual ratios of the samples for HNO solutions up to 3 M were found to be decreased identically, where ca. 20% of NBP was degraded after the irradiation of 1 MGy. It was found that the degradation of the samples of 6 M HNO
solutions up to 3 M were found to be decreased identically, where ca. 20% of NBP was degraded after the irradiation of 1 MGy. It was found that the degradation of the samples of 6 M HNO is more distinguished, where ca. 30% was degraded after the irradiation of 0.1 MGy. As the result of the investigation of the degradation mechanism of NBP, it was revealed that the degradation started from the cleavage of the pyrrolidone ring of NBP by the addition of oxygen atom, followed by the formation of chain monoamides and C4 compounds by the continuous addition of oxygen, leading to the generation of oxalic acid.
is more distinguished, where ca. 30% was degraded after the irradiation of 0.1 MGy. As the result of the investigation of the degradation mechanism of NBP, it was revealed that the degradation started from the cleavage of the pyrrolidone ring of NBP by the addition of oxygen atom, followed by the formation of chain monoamides and C4 compounds by the continuous addition of oxygen, leading to the generation of oxalic acid.
Morita, Yasuji; Takao, Koichiro*; Kim, S.-Y.; Kawata, Yoshihisa; Harada, Masayuki*; Nogami, Masanobu*; Nishimura, Kenji*; Ikeda, Yasuhisa*
Journal of Nuclear Science and Technology, 46(12), p.1129 - 1136, 2009/12
Times Cited Count:18 Percentile:74.76(Nuclear Science & Technology)A reprocessing system for spent FBR fuels consisting of two precipitation processes has been proposed. In this system, first only U(IV) species are precipitated using pyrrolidone derivative with low hydrophobicity and donicity, and secondly residual U(VI) and Pu(IV, VI) are precipitated simultaneously using pyrrolidone derivative with high precipitation ability. In this study, we have examined precipitation behavior of U(VI), Pu(IV), and Pu(VI) species in nitric acid solutions by using  -
- -propyl-2-pyrrolidone (NProP),
-propyl-2-pyrrolidone (NProP),  -butyl-2-pyrrolidone (NBP),
-butyl-2-pyrrolidone (NBP),  -
-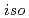 -butyl-2-pyrrolidone (NiBP), or
-butyl-2-pyrrolidone (NiBP), or  -cyclohexyl-2-pyrrolidone (NCP) to select the precipitants for the first precipitation process. As a result, NBP were found to be the most promising precipitant for the first precipitation process.
-cyclohexyl-2-pyrrolidone (NCP) to select the precipitants for the first precipitation process. As a result, NBP were found to be the most promising precipitant for the first precipitation process.
 -Alkyl-2-pyrrolidone derivatives (Alkyl =
-Alkyl-2-pyrrolidone derivatives (Alkyl =  -propyl,
-propyl,  -butyl,
-butyl, 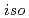 -butyl, and cyclohexyl)
-butyl, and cyclohexyl)Takao, Koichiro*; Noda, Kyoko*; Nogami, Masanobu*; Sugiyama, Yuichi*; Harada, Masayuki*; Morita, Yasuji; Nishimura, Kenji*; Ikeda, Yasuhisa*
Journal of Nuclear Science and Technology, 46(10), p.995 - 999, 2009/10
Times Cited Count:14 Percentile:66.94(Nuclear Science & Technology)We have been developing an advanced reprocessing system for spent FBR fuels based on precipitation method using pyrrolidone derivatives. In the present study, the solubility of UO (NO
(NO )
) (NRP)
(NRP) (NRP =
(NRP =  -alkyl-2-pyrrolidone, alkyl =
-alkyl-2-pyrrolidone, alkyl =  -propyl,
-propyl,  -butyl,
-butyl, 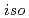 -butyl and cyclohexyl) in aqueous solutions with HNO
-butyl and cyclohexyl) in aqueous solutions with HNO has been examined. As a result, the solubility of each species of UO
has been examined. As a result, the solubility of each species of UO (NO
(NO )
) (NRP)
(NRP) generally decreases with increasing concentrations of HNO
generally decreases with increasing concentrations of HNO and NRP (
and NRP (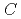 (HNO
(HNO ) and
) and 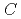 (NRP), respectively). The solubility of UO
(NRP), respectively). The solubility of UO (NO
(NO )
) (NRP)
(NRP) also depends on the type of NRP; a higher hydrophobicity of NRP generally leads to a lower solubility of UO
also depends on the type of NRP; a higher hydrophobicity of NRP generally leads to a lower solubility of UO (NO
(NO )
) (NRP)
(NRP) . The logarithms of effective solubility products (
. The logarithms of effective solubility products (
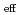 ) of UO
) of UO (NO
(NO )
) (NRP)
(NRP) at different
at different 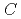 (HNO
(HNO ) values and 293 K were evaluated.
) values and 293 K were evaluated.
 -alkylated 2-pyrrolidone derivatives; Design and optimization of promising precipitant for uranyl ion
-alkylated 2-pyrrolidone derivatives; Design and optimization of promising precipitant for uranyl ionTakao, Koichiro*; Noda, Kyoko*; Morita, Yasuji; Nishimura, Kenji*; Ikeda, Yasuhisa*
Crystal Growth & Design, 8(7), p.2364 - 2376, 2008/07
Times Cited Count:35 Percentile:89.88(Chemistry, Multidisciplinary)Molecular and crystal structures of UO (NO
(NO )
) (NRP)
(NRP) (NRP = N-alkylated 2-pyrrolidone derivative) have been investigated by using single crystal X-ray analysis, IR and Raman spectroscopies. All UO
(NRP = N-alkylated 2-pyrrolidone derivative) have been investigated by using single crystal X-ray analysis, IR and Raman spectroscopies. All UO (NO
(NO )
) (NRP)
(NRP) complexes have typical structural properties of UO
complexes have typical structural properties of UO (NO
(NO )
) (L)
(L) (L = unidentate ligand), i.e., hexagonal-bipyramidal geometry, two NRP and two NO
(L = unidentate ligand), i.e., hexagonal-bipyramidal geometry, two NRP and two NO
 located in trans positions in an equatorial plane of the uranyl ion. Observation of the crystals of the uranyl nitrate complexes with N-n-propyl-2-pyrrolidone and N-iso-propyl-2-pyrrolidone indicate the presence of significant voids in the crystal lattices of these compounds. From this result, an approach for construction of efficient packing of UO
located in trans positions in an equatorial plane of the uranyl ion. Observation of the crystals of the uranyl nitrate complexes with N-n-propyl-2-pyrrolidone and N-iso-propyl-2-pyrrolidone indicate the presence of significant voids in the crystal lattices of these compounds. From this result, an approach for construction of efficient packing of UO (NO
(NO )
) (NRP)
(NRP) was proposed. Consequently, it was found that N-iso-butyl-2-pyrrolidone completely satisfies with the requirement for the efficient packing by filling the voids with the alkyl chain.
was proposed. Consequently, it was found that N-iso-butyl-2-pyrrolidone completely satisfies with the requirement for the efficient packing by filling the voids with the alkyl chain.
Harada, Masayuki*; Ikeda, Yasuhisa*; Asakura, Toshihide; Morita, Yasuji; Nogami, Masanobu*; Nishimura, Kenji*
no journal, ,
Precipitation behavior of U(VI), Pu(IV) and Pu(VI) with pyrrolidone derivatives of N-butyl-2-pyrrolidone (NBP) and N-propyl-2-pyrrolidone (NProP) was examined to develop a simple and economical reprocessing process of spent nuclear fuel based only on precipitation method. These precipitants have lower donicity and hydrophobicity than N-cyclohexyl-2-pyrrolidone (NCP) which has already been examined for U(VI) precipitation. The addition of twice amount of the precipitant with molar ratio to U(VI) gave 70% precipitation of U(VI) from nitric acid solution of 2M U. Experiments on Pu precipitation behavior revealed that both NBP and NProP have lower ability to make precipitates of Pu(IV) and Pu(VI), which would contribute to the development of the efficient precipitation step only for U(VI).
Maruyama, Koichi*; Nogami, Masanobu*; Ikeda, Yasuhisa*; Nishimura, Kenji*; Morita, Yasuji
no journal, ,
Radiation and heat resistance of low coordinate and hydrophobic pyrrolidone derivatives, NProP and NBP, which are selective precipitants for U(VI), was examined by  -irradiation and heating of the 3M nitric and solutions of the precipitants. Test results of radiation resistance of NProP and NBP showed that the ability to make precipitate of U(VI) was kept until 0.5 MGy of irradiation. After the heating of the 3M nitric and solutions of NProP and NBP at 50
-irradiation and heating of the 3M nitric and solutions of the precipitants. Test results of radiation resistance of NProP and NBP showed that the ability to make precipitate of U(VI) was kept until 0.5 MGy of irradiation. After the heating of the 3M nitric and solutions of NProP and NBP at 50  C, the molecular structures of NBP and NProP were changed within 4 days and 10days, respectively. It was found that the change of the structure does not weaken but reinforce the ability to make precipitate of U(VI), which would due to the increase of the hydrophobicity.
C, the molecular structures of NBP and NProP were changed within 4 days and 10days, respectively. It was found that the change of the structure does not weaken but reinforce the ability to make precipitate of U(VI), which would due to the increase of the hydrophobicity.
Harada, Masayuki*; Asakura, Toshihide; Morita, Yasuji; Nogami, Masanobu*; Nishimura, Kenji*; Ikeda, Yasuhisa*
no journal, ,
Pyrrolidone derivatives of N-propyl-2-pyrrolidone (NProP) and N-butyl-2-pyrrolidone (NBP) are being studied for selective precipitants for uranyl ion. Structural analyses of UO (NO
(NO )
) L
L [L=NProP or NBP] was carried out using X-ray diffraction method. Relationship of the structure of the complex and the precipitation ability of the reagent are also discussed considering results of Raman spectrum measurement.
[L=NProP or NBP] was carried out using X-ray diffraction method. Relationship of the structure of the complex and the precipitation ability of the reagent are also discussed considering results of Raman spectrum measurement.
Harada, Masayuki*; Morita, Yasuji; Nogami, Masanobu*; Nishimura, Kenji*; Ikeda, Yasuhisa*
no journal, ,
In the present study, we have examined the precipitation ability of N-butyl-2-pyrrolidone (NBP) and N-propyl-2-pyrrolidone (NProP) as selective precipitants for uranyl ion, which have lower hydrophobicity than N-cycrohexyl-2-pyrrolidone (NCP) we had studied previously. As a result, we found that NBP and NProP can precipitate uranyl ions stoichiometrically regardless of the concentrations of uranyl ion and nitric acid, and the temperature of solutions. Furthermore, in the Pu concentration range of 0.04 to 0.06 M, Pu(IV) and Pu(VI) species were not precipitated by NProP, while Pu(IV) species are precipitated with addition of excess amount of NBP. From these results, it is expected that in the first precipitation process we can precipitate uranyl ions without coprecipitation of Pu(IV) species by using NBP or NProP.
Kim, S.-Y.; Kawata, Yoshihisa; Morita, Yasuji; Ikeda, Yasuhisa*; Nishimura, Kenji*
no journal, ,
Precipitation behavior of Pu with pyrrolidone derivatives of N-n-butyl-2-pyrrolidone (NBP), N-propyl-2-pyrrolidone (NProP) and N-iso-butyl-2-pyrrolidone (NiBP) in the solutions of only Pu and U-Pu mixture has been examined in order to evaluate their applicability to the selective U precipitation process in the reprocessing based only on precipitation method. We have previously developed a process with N-cyclohexyl-2-pyrrolidone (NCP). It was found that the precipitation ability of NBP, NProP and NiBP is lower than that of NCP and the lower co-precipitation and faster re-dissolution of Pu(IV) in the mixed solution of U(VI) and Pu(IV) was obtained in the precipitation with NBP, NProP and NiBP than with NCP. From these results, it is expected that those new precipitants would make the selective U precipitation process more selective and effective.
Morita, Yasuji; Kim, S.-Y.; Kawata, Yoshihisa; Sato, Makoto; Ikeda, Yasuhisa*; Takao, Koichiro*; Noda, Kyoko*; Nishimura, Kenji*
no journal, ,
Precipitation behavior of Pu with pyrrolidone derivatives of N-(1,2-dimethyl)propyl-2-pyrrolidone (NDMProP) and N-neopenthyl-2-pyrrolidone (NNpP) in the solutions of U-Pu mixture has been examined in order to evaluate their applicability to the U-Pu co-precipitation process in the reprocessing based only on precipitation method. We have previously developed a process with N-cyclohexyl-2-pyrrolidone (NCP). It was found that U(VI) was precipitated in a high yield with any of the three precipitants and the precipitation yield of Pu(IV) was increased with the added amount of the precipitants. When NNpP was added with the ratio of [NNpP]/[U+Pu]=2.5, 99.5% of Pu was precipitate. Since NNpP showed the highest precipitation ability for Pu(IV) and the best physical property as precipitate, NNpP would be the most appropriate precipitant for the U-Pu co-precipitation process.
Takao, Koichiro*; Noda, Kyoko*; Nogami, Masanobu*; Sugiyama, Yuichi*; Harada, Masayuki*; Ikeda, Yasuhisa*; Morita, Yasuji; Nishimura, Kenji*
no journal, ,
Precipitation behavior of U(VI) and some fission products (FP) with pyrrolidone derivatives of N-(1,2-dimethyl)propyl-2-pyrrolidone (NDMProP) and N-neopenthyl-2-pyrrolidone (NNpP) in the solutions of U-FP mixture has been examined in order to evaluate their applicability to the U-Pu co-precipitation process in the reprocessing based only on precipitation method. We have previously developed a process with N-cyclohexyl-2-pyrrolidone (NCP). It was found that U(VI) was precipitated in a high yield with any of the three precipitants and the order of the precipitation ability for U(VI) was NCP NNpP
NNpP NDMProP. Decontamination factors (DF) against FP except Zr(IV) and Mo(VI) in the U(VI) precipitation were over 100, and therefore NNpP and NDMProP can be used as an alternative precipitants to NCP.
NDMProP. Decontamination factors (DF) against FP except Zr(IV) and Mo(VI) in the U(VI) precipitation were over 100, and therefore NNpP and NDMProP can be used as an alternative precipitants to NCP.
Nogami, Masanobu*; Noda, Kyoko*; Takao, Koichiro*; Sugiyama, Yuichi*; Harada, Masayuki*; Ikeda, Yasuhisa*; Kawata, Yoshihisa; Morita, Yasuji; Nishimura, Kenji*
no journal, ,
We have been developing an advanced reprocessing system for spent FBR fuels based on precipitation method using pyrrolidone derivatives. The present study deals with durability of new precipitants with low and high hydrophobicity against  -irradiation and heating. Results showed that the precipitants with low hydrophobicity, N-n-butyl-2-pyrrolidone, N-propyl-2-pyrrolidone and N-iso-butyl-2-pyrrolidone, have enough durability against
-irradiation and heating. Results showed that the precipitants with low hydrophobicity, N-n-butyl-2-pyrrolidone, N-propyl-2-pyrrolidone and N-iso-butyl-2-pyrrolidone, have enough durability against  -irradiation. The precipitants with high hydrophobicity, N-cyclohexyl-2-pyrrolidone, N-(1,2-dimethyl)propyl-2-pyrrolidone and N-neopenthyl-2-pyrrolidone, also have enough durability but gave lower precipitation yield when they irradiated with higher dose rate. The precipitation ability of all the precipitants did not changed by the heating at 50
-irradiation. The precipitants with high hydrophobicity, N-cyclohexyl-2-pyrrolidone, N-(1,2-dimethyl)propyl-2-pyrrolidone and N-neopenthyl-2-pyrrolidone, also have enough durability but gave lower precipitation yield when they irradiated with higher dose rate. The precipitation ability of all the precipitants did not changed by the heating at 50 C for three days.
C for three days.
Kim, S.-Y.; Haga, Yoshinori; Yamamoto, Etsuji; Kawata, Yoshihisa; Morita, Yasuji; Ikeda, Yasuhisa*; Nishimura, Kenji*
no journal, ,
The crystal structure of plutonyl(VI) nitrate complexes, PuO (NO
(NO )
) (L)
(L) [L=N-cyclohexyl-2-pyrrolidone (NCP), N-Neopentyl-2-pyrrolidone (NNpP)] have been prepared by mixing PuO
[L=N-cyclohexyl-2-pyrrolidone (NCP), N-Neopentyl-2-pyrrolidone (NNpP)] have been prepared by mixing PuO (NO
(NO )
) and L in an aqueous nitric acid solution and its crystal structure was determined using X-ray diffractometry and confirmed by IR spectroscopy. The plutonyl(VI) nitrate complexes with L normally have a composition of PuO
and L in an aqueous nitric acid solution and its crystal structure was determined using X-ray diffractometry and confirmed by IR spectroscopy. The plutonyl(VI) nitrate complexes with L normally have a composition of PuO (NO
(NO )
) (L)
(L) , which shows common structural features, i.e., the plutonyl ion is surrounded by four oxygen atoms from two NO
, which shows common structural features, i.e., the plutonyl ion is surrounded by four oxygen atoms from two NO
 and two donating atoms of two L in its equatorial plane. X-ray diffraction study reveals that average distances between plutonium atom and plutonyl-, nitrate-, and carbonyl- oxygen atoms are 1.727-1.731
and two donating atoms of two L in its equatorial plane. X-ray diffraction study reveals that average distances between plutonium atom and plutonyl-, nitrate-, and carbonyl- oxygen atoms are 1.727-1.731  , 2.490-2.507
, 2.490-2.507  , 2.377-2.390
, 2.377-2.390  , respectively.
, respectively.
Nogami, Masanobu*; Takao, Koichiro*; Sugiyama, Yuichi*; Noda, Kyoko*; Harada, Masayuki*; Ikeda, Yasuhisa*; Morita, Yasuji; Nishimura, Kenji*
no journal, ,
We have been developing an advanced reprocessing system for spent FBR fuels based on precipitation method using pyrrolidone derivatives. The present study deals with the effect of addition of masking agents on co-precipitation of Pu(IV) in the selective U(VI) precipitation. It was found that precipitation of U(IV), which was used as substitute for Pu(IV), was suppressed by the presence of acetohydoxamic acid.
Nogami, Masanobu*; Kawasaki, Takeshi*; Takao, Koichiro*; Noda, Kyoko*; Sugiyama, Yuichi*; Harada, Masayuki*; Ikeda, Yasuhisa*; Morita, Yasuji; Chikazawa, Takahiro*; Kikuchi, Toshiaki*; et al.
no journal, ,
A reprocessing system for spent FBR fuels based on the two precipitation processes has been proposed. In this system, first only U(VI) species are precipitated using pyrrolidone derivatives (NRP) with low hydrophobicity and donicity, and secondly residual U(VI) and Pu(IV, VI) are precipitated simultaneously using pyrrolidone derivative with high precipitation ability. In order to develop such a reprocessing method, behavior of uranyl ions in HNO has been studied using various novel NRP. In this presentation, recent advances on precipitation behavior and of uranyl ions and calcination of U(VI)-NRP will be introduced.
has been studied using various novel NRP. In this presentation, recent advances on precipitation behavior and of uranyl ions and calcination of U(VI)-NRP will be introduced.
Kim, S.-Y.; Kawata, Yoshihisa; Morita, Yasuji; Nogami, Masanobu*; Harada, Masayuki*; Ikeda, Yasuhisa*; Kikuchi, Toshiaki*; Nishimura, Kenji*
no journal, ,
A reprocessing system for spent FBR fuels based on the two precipitation processes has been proposed. In this system, first only U(VI) species are precipitated using pyrrolidone derivatives (NRP) with low hydrophobicity and donicity, and secondly residual U(VI) and Pu(IV, VI) are precipitated simultaneously using pyrrolidone derivative with high precipitation ability. It is important to control precipitation of Pu(IV), and recent advances on precipitation behavior of Pu(IV, VI) by using novel pyrrolidone derivatives will be introduced in this presentation. Precipitation tests using mixed solutions of U(VI) and Pu(IV) showed that N-n-butyl-2-pyrrolidone (NBP) is the most appropriate precipitant for the first precipitation step and N-neopenthyl-2-pyrrolidone (NNpP) is the most appropriate precipitant for the second step.