Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 (C
(C H
H O
O )
) (C
(C H
H N)
N)Nakamura, Akio; Nakada, Masami; Nakamoto, Tadahiro*; Kitazawa, Takafumi*; Takeda, Masuo*
Journal of Magnetism and Magnetic Materials, 310(2, Part2), p.1447 - 1449, 2007/03
Magnetic property of a nitrogen (N)-coordinated neptunyl (+2) (Np(VI)) complex. 1 NpO (acac)
(acac) (py) (acac=C
(py) (acac=C H
H O
O , py=C
, py=C H
H N) was studied by DC magnetization (M) measurements. While previous
N) was studied by DC magnetization (M) measurements. While previous  Np M
Np M ssbauer data of 1 exhibited para magnetic-relaxation spectra similar to other oxo-neptunyls (+1,+2), the DC-
ssbauer data of 1 exhibited para magnetic-relaxation spectra similar to other oxo-neptunyls (+1,+2), the DC- data have revealed many strikingly different features of 1 from the latter: first, its reciprocal susceptibility (
data have revealed many strikingly different features of 1 from the latter: first, its reciprocal susceptibility (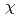
 ) vs.
) vs.  (K) plots showed a peculiar strongly field (
(K) plots showed a peculiar strongly field (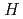 )-dependent nonparamagnetic behavior, which suggests the presence of small ferromagnetic component with
)-dependent nonparamagnetic behavior, which suggests the presence of small ferromagnetic component with 
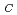
 300 K. In addition, its low-temperature isothermal
300 K. In addition, its low-temperature isothermal  exhibited anomalous relaxation behavior involving initial over-responsive
exhibited anomalous relaxation behavior involving initial over-responsive  change and subs equent drift back to its "equilibrium" value (
change and subs equent drift back to its "equilibrium" value (
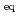 ). Moreover, such
). Moreover, such 
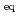 showed strong perturbation effect from the SQUID-
showed strong perturbation effect from the SQUID- measurement itself. Such novel magnetic features suggest the presence of a largely metastable fluctuating magnetic (spin plus orbital) state in 1 as a collective body of individual Ising-type neptunyl (O=Np=O)
measurement itself. Such novel magnetic features suggest the presence of a largely metastable fluctuating magnetic (spin plus orbital) state in 1 as a collective body of individual Ising-type neptunyl (O=Np=O)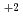 molecular magnets.
molecular magnets.
Saeki, Masakatsu; Nakada, Masami; Kawasaki, Takeshi*; Nishimura, Tatsuru*; Kitazawa, Takafumi*; Takeda, Masuo*
Journal of Radioanalytical and Nuclear Chemistry, 270(2), p.379 - 384, 2006/11
Times Cited Count:3 Percentile:24.11(Chemistry, Analytical)no abstracts in English
 (C
(C H
H O
O )
) (C
(C H
H N)
N)Nakamura, Akio; Nakada, Masami; Nakamoto, Tadahiro*; Kitazawa, Takafumi*; Takeda, Masuo*
Journal of the Physical Society of Japan, 75(Suppl.), p.146 - 148, 2006/08
no abstracts in English
Sato, Kenji*
JNC TY9400 2005-005, 44 Pages, 2005/03
As part of studies for phenomenological investigation of sodium droplet burning behavior, in our previous experimental studies for suspended single sodium droplet, behavior of ignition process and succeeding combustion, ignition delay time, and droplet temperature history had been investigated. In this study, combustion experiments of suspended sodium droplet were performed in upward dry air flow by expanding the range of free-stream velocity U of air flow into 400 cm/s with initial droplet temperature Ti = 300, 350, and 400 C and initial droplet diameter 4 mm at first. Then, the combustion experiments were also performed by changing the initial droplet diameter from 2.3 to 4.4 mm with Ti = 350 and 400
C and initial droplet diameter 4 mm at first. Then, the combustion experiments were also performed by changing the initial droplet diameter from 2.3 to 4.4 mm with Ti = 350 and 400 C and U = 100 cm/s. From the experimental results, the effects of free-stream velocity, initial droplet temperature, and initial droplet diameter on the ignition/burning behavior and ignition delay time were examined. The obtained results are as follows: (1) Ignition phenomena of suspended droplet were observed for all examined experimental conditions up to 400 cm/s. The orange emission observed at the moment of ignition occurs simultaneously over whole droplet surface except the top region of it. (2) The feature of the dependence of ignition delay time on the free-stream velocity is independent of the initial droplet temperature. With the increase of the free-stream velocity, up to 300 cm/s the ignition delay time decreases with decreasing dependency, and then the dependency increases more. (3) The ignition delay time increases with the increase of initial droplet diameter. The dependency increases as the initial droplet diameter increases. The ignition delay time extrapolated toward zero diameters from the obtained results becomes to be essentially zero.
C and U = 100 cm/s. From the experimental results, the effects of free-stream velocity, initial droplet temperature, and initial droplet diameter on the ignition/burning behavior and ignition delay time were examined. The obtained results are as follows: (1) Ignition phenomena of suspended droplet were observed for all examined experimental conditions up to 400 cm/s. The orange emission observed at the moment of ignition occurs simultaneously over whole droplet surface except the top region of it. (2) The feature of the dependence of ignition delay time on the free-stream velocity is independent of the initial droplet temperature. With the increase of the free-stream velocity, up to 300 cm/s the ignition delay time decreases with decreasing dependency, and then the dependency increases more. (3) The ignition delay time increases with the increase of initial droplet diameter. The dependency increases as the initial droplet diameter increases. The ignition delay time extrapolated toward zero diameters from the obtained results becomes to be essentially zero.
 Np M
Np M essbauer parameter and crystal structure of neptunyl compounds
essbauer parameter and crystal structure of neptunyl compoundsSaeki, Masakatsu
Nihon Kessho Gakkai-Shi, 46(6), p.415 - 420, 2004/12
no abstracts in English
Sato, Kenji*
JNC TY9400 2004-022, 45 Pages, 2004/03
As part of studies for phenomenological investigation of sodium droplet burning behavior, in our previous experimental studies for suspended single sodium droplet, behavior of ignition process and succeeding combustion, ignition delay time, and droplet temperature history had been investigated. In the present study, by using 4 mm diam. suspended sodium droplet, combustion experiments were performed for extended free-stream velocity range of dry air up to 200 cm/s, and for the initial droplet temperatures Ti = 300 deg C and 400 deg C, and the effects of the free-stream velocity and initial droplet temperature on the ignition/burning behavior and ignition delay time were examined by using high speed video camera. The obtained experimental results are as follows: (1)Ignition phenomena of suspended spherical shape droplet were observed for all examined experimental conditions except the case of free-stream velocity U = 200 cm/s at 300 deg C, where detachment of droplet from the support due to strained oxide film occurred. (2)The ignition delay time defined as the time to evolution of orange-light emitting zone or flame zone decreases with the increase of the free-stream velocity or of initial droplet temperature. Examples of typical ignition delay time are 0.68 s at U = 20 cm/s, 0.52 s at U = 100 cm/s, and 0.37 s at 200 cm/s for Ti = 400 deg C. (3)The orange-light emission at the moment of ignition occurs simultaneously over whole surface except the top region of the droplet, The intensity of the emission at the moment of ignition takes its maximum at the bottom region or upstream region of the droplet, and the emission intensity during the stable burning period increases with the increase of U. (4)When Ti is 300 deg C, formation of temporal multiple short projections are observed before ignition for all examined free-stream velocities. The projections often do not disappear before ignition when the velocity is relatively high. (5)The layer or cloud composed of aerosol
 O
O -ZrO
-ZrO system
systemWang, J.*; Otobe, Haruyoshi; Nakamura, Akio; Takeda, Masuo*
Journal of Solid State Chemistry, 176(1), p.105 - 110, 2003/11
Times Cited Count:14 Percentile:44.29(Chemistry, Inorganic & Nuclear)no abstracts in English
 O
O -ZrO
-ZrO system xGdO
system xGdO -(1-x)ZrO
-(1-x)ZrO with 0.18
with 0.18  x
x  0.62
0.62Wang, J.*; Nakamura, Akio; Takeda, Masuo*
Solid State Ionics, 164(3-4), p.185 - 191, 2003/11
Times Cited Count:34 Percentile:79.32(Chemistry, Physical)no abstracts in English
Sato, Kenji*
JNC TY9400 2004-003, 51 Pages, 2003/06
As part of studies for phenomenological investigation of sodium droplet burning behavior, in our previous experimental studies, ignition process and succeeding combustion of suspended single sodium droplet had been investigated by using high speed movie camera, and a temperature measurement system feasible for the experiment had been developed. In the present study, by using 4 mm diam. suspended sodium droplet, combustion experiments were performed for the free-stream velocity of dry air flow of 20 to 60 cm/s, and for the initial droplet temperature of 280 to 400deg-C, and the effects of the free-stream velocity and initial droplet temperature on the ignition behavior and droplet temperature variation with time were examined by using high speed movie camera and sheath-type fine thermocouple. The experimental results are as follows: (1)When the initial droplet temperature is less than 290 deg-C, before ignition the oxide film accompanied with vertical streak appeared and the droplet turned to teardrop shape. (2)The ignition delay time defined as the time to evolution of orange color light emission zone or flame zone decreases with the increase of the free-stream velocity or of initial droplet temperature. Examples of typical ignition time are 1.4 s at the free-stream velocity 20 cm/s and initial droplet temperature 300 deg-C, and 0.65 s at 60 cm/s and 400 deg-C. (3)The dependence of the ignition delay time on the free-stream velocity decreases as the free stream velocity increases. (4)The droplet temperatures at the moment of melting extending all over the surface and at the moment of ignition are around 460 deg-C and 500 deg-C to 600 deg-C (mostly around 575 deg-C), respectively. These values are essentially independent of the free-stream velocity and initial droplet temperature. (5)The rate of temperature rise does not change through the moment of ignition. (6)The asymptotic droplet temperature at approaching to quasi-steady combustion state following ignition is i
Sato, Kenji*
JNC TY9400 2003-008, 17 Pages, 2003/03
Experimental studies have been performed since JFY1997 for the combustion of suspended sodium droplet in order to understand the detailed phenomenology of its ignition and burning behavior. The author has accumulated to data the high-speed camera picture data of change in a droplet surface and its neighboring gas phase of spontaneous ignition of suspended sodium doroplet in air flow, after establishing fundamental experimental techniques to observe the process. In the study from JFY2000 to 2001, a system was developed which enable to measure the temperature of sodium droplet and of flame zone. The study includes the improvement of droplet generation system so as to prevent the oxidation of suspended droplet. The temperature measurement system consists of silica-coated fine R-type thermocouple of 20  m in diam. adopted to ensure excellent responsibility, and its driving apparatus. The driving system is composed of pulse motor, its controller and delay circuit, and its performance was confirmed by several tests. It was shown from the preliminary tests that the basic technique was established for detailed temperature measurment around the time of droplet ignition, though several problems should be investigated furthermore such as electrical noise removal in temperature measurment and effect of thermocouple coating.
m in diam. adopted to ensure excellent responsibility, and its driving apparatus. The driving system is composed of pulse motor, its controller and delay circuit, and its performance was confirmed by several tests. It was shown from the preliminary tests that the basic technique was established for detailed temperature measurment around the time of droplet ignition, though several problems should be investigated furthermore such as electrical noise removal in temperature measurment and effect of thermocouple coating.
*
JNC TJ9400 99-011, 57 Pages, 1999/10
For appropriate understanding and/or prediction of the combustion behavior of sodium, working as liquid coolant in fast breeder reactors, in case of leakage accident, phenomenological analyses of the behavior must be also important along with conventional engineering approach. Following our previous study in the last year, the major objective of this experimental research is to elucidate the effects of the initial temperature and diameter of droplet, and of the air flow velocity on ignition process of a sodium droplet, by exposing a suspended droplet to the air flow at room-temperature. In the experiments, a high-temperature droplet suspended from the end of a fine stainless steel nozzle of the liquid sodium supply system was exposed to an upward air flow, and the ignition and succeeding combustion phenomena were observed by using high-speed color video recording system. In the preliminary study, the effects of lighting and image data processing on obtaining pictures suitable to analyses were investigated with the apparatus used in the previous study. After the experimental apparatus was modified partially in order to expose the unreacted droplet to the air flow more quickly, main experiments were performed in synthetic dry air or oxygen-nitrogen mixture of 21% oxygen. Good quality pictures of the phenomena achieved under good conditions were recorded even fbr a few cases. The details of the ignition process of a sodium droplet, including the aspects of the surface and light emission, were examined, and the effects of the air flow velocity were discussed. Since number of performed experimental runs was small, the effect of the initial droplet temperature were not examined.
Sato, Kenji*
PNC TJ9807 98-001, 53 Pages, 1998/08
None
Sato, Kenji*; Takekuma, Akiko*
no journal, ,
no abstracts in English
 -oxide
-oxideEguchi, Ayano; Morita, Kotaro*; Okamura, Hiroyuki; Hirayama, Naoki*
no journal, ,
2-Mercaptopyridine  -oxide (HSPyO) is a bidentate ligand with a hard O-donor and a soft S-donor. This ligand is expected to be effective in an ionic liquid (IL) chelate extraction because of its high solubility in ILs. In this study, we investigated the IL chelate extraction behavior of Al(III), Ga(III), and In(III) into ILs, 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imides ([C
-oxide (HSPyO) is a bidentate ligand with a hard O-donor and a soft S-donor. This ligand is expected to be effective in an ionic liquid (IL) chelate extraction because of its high solubility in ILs. In this study, we investigated the IL chelate extraction behavior of Al(III), Ga(III), and In(III) into ILs, 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imides ([C mim][Tf
mim][Tf N], n = 2, 4, 8), using HSPyO as an extractant. The extraction selectivity order was In(III)
N], n = 2, 4, 8), using HSPyO as an extractant. The extraction selectivity order was In(III)  Ga(III), whereas Al(III) was not extracted. Therefore, HSPyO was favorable for the extraction of soft In(III). The extractability order for solvents was ILs
Ga(III), whereas Al(III) was not extracted. Therefore, HSPyO was favorable for the extraction of soft In(III). The extractability order for solvents was ILs  chloroform in the Ga(III) extraction, indicating that ILs exhibit high extraction performance. In addition, it was implied that the extracted species were the neutral complexes, M(SPyO)
chloroform in the Ga(III) extraction, indicating that ILs exhibit high extraction performance. In addition, it was implied that the extracted species were the neutral complexes, M(SPyO) , for all metals.
, for all metals.
Eguchi, Ayano; Morita, Kotaro*; Okamura, Hiroyuki; Hirayama, Naoki*
no journal, ,
In chelate extraction of metal ions into conventional organic solvents, the metals are generally extracted as uncharged complexes. Moreover, coordinatively saturated (unhydrated) complexes are preferable as extracted species. In ionic liquid (IL) chelate extraction, on the contrary, also charged species or coordinatively unsaturated (hydrated) species can be extracted. In this study, we investigated the IL chelate extraction behavior of trivalent metal ions, Fe(III), Al(III), Ga(III) and In(III), into 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imides ([C mim][Tf
mim][Tf N], n = 2, 4, 8) using 8-quinolinol (H
N], n = 2, 4, 8) using 8-quinolinol (H Q
Q ) and 2-mercaptopyridine
) and 2-mercaptopyridine  -oxide (H
-oxide (H SPyO
SPyO ) as bidentate ligands. In the case of H
) as bidentate ligands. In the case of H Q
Q , only uncharged M
, only uncharged M Q
Q
 was extracted into chloroform, whereas coordinatively unsaturated cationic complex [M
was extracted into chloroform, whereas coordinatively unsaturated cationic complex [M Q
Q
 ]
] was also extracted into ILs by ion exchange. In the case of H
was also extracted into ILs by ion exchange. In the case of H SPyO
SPyO , it was implied that uncharged M
, it was implied that uncharged M (SPyO)
(SPyO)
 was extracted into both chloroform and ILs. Therefore, the extracted species depends on the ligands in ILs.
was extracted into both chloroform and ILs. Therefore, the extracted species depends on the ligands in ILs.
Eguchi, Ayano; Okamura, Hiroyuki; Sugita, Tsuyoshi; Ueda, Yuki; Morita, Kotaro*; Shimojo, Kojiro; Naganawa, Hirochika; Hirayama, Naoki*
no journal, ,
In this study, the effect of the hydrophobicity and structure of an ionic liquid (IL) anion on the extraction of trivalent lanthanoid ions (Ln(III) = La, Nd, Eu, Dy, Lu) using 2-thenoyltrifluoroacetone (Htta) was investigated. Four 1-butyl-3-methylimidazolium bis(perfluoroalkanesulfonyl)imides with different perfluoroalkyl (Rf) chain length (n=0, 1, 2, 4) were synthesized and used as the extraction solvent. The extraction selectivity of Ln(III) was in the order of Lu 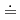 Dy
Dy  Eu
Eu  Nd
Nd  La for all ILs. In addition, there was no significant difference in the extractability among ILs. The extracted species of each Ln(III) in these IL systems were investigated based on the slope of the plots of the logarithmic distribution ratio of Ln(III) as a function of aqueous phase pH. The results implied that the extracted species can be adjusted by Rf chain length of IL anions.
La for all ILs. In addition, there was no significant difference in the extractability among ILs. The extracted species of each Ln(III) in these IL systems were investigated based on the slope of the plots of the logarithmic distribution ratio of Ln(III) as a function of aqueous phase pH. The results implied that the extracted species can be adjusted by Rf chain length of IL anions.
Eguchi, Ayano; Morita, Kotaro*; Okamura, Hiroyuki; Hirayama, Naoki*
no journal, ,
In chelate extraction of metal ions into organic solvents, formation of their uncharged complexes with ligands is required. Moreover, coordinatively saturated (unhydrated) complexes are more preferable as extracted species. On the other hand, in ionic liquid chelate extraction, ionic liquid can extract charged species and coordinatively unsaturated (hydrated) species. In this study, the effects of bidentate ligands on ionic liquid chelate extraction of trivalent metal ions, Fe(III), Al(III), Ga(III) and In(III), into 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imides ([C mim][Tf
mim][Tf N], n=2,4,8) was studied using 8-quinolinol (HQ) and 2-mercaptopyridine
N], n=2,4,8) was studied using 8-quinolinol (HQ) and 2-mercaptopyridine  -oxide (HSPyO) as extractants. The extraction selectivity order for trivalent metal ions was Fe
-oxide (HSPyO) as extractants. The extraction selectivity order for trivalent metal ions was Fe 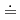 Ga
Ga  In
In  Al for HQ and Fe
Al for HQ and Fe  In
In  Ga
Ga 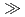 Al for HSPyO. Namely, HSPyO was favorable for extraction of In(III). The extractability of Ga(III) was in the order of [C
Al for HSPyO. Namely, HSPyO was favorable for extraction of In(III). The extractability of Ga(III) was in the order of [C mim][Tf
mim][Tf N]
N]  [C
[C mim][Tf
mim][Tf N]
N]  [C
[C mim][Tf
mim][Tf N]
N]  chloroform for HSPyO, whereas reversed order was obtained for HQ. These results were seemed to be based on difference in concentration of deprotonated bidentate ligands in water.
chloroform for HSPyO, whereas reversed order was obtained for HQ. These results were seemed to be based on difference in concentration of deprotonated bidentate ligands in water.
Eguchi, Ayano; Okamura, Hiroyuki; Sugita, Tsuyoshi; Ueda, Yuki; Morita, Kotaro*; Shimojo, Kojiro; Naganawa, Hirochika; Hirayama, Naoki*
no journal, ,
In this study, the effect of the perfluoroalkyl (Rf) chain length of the ionic liquid (IL) anion on the extraction of trivalent lanthanoid ions (Ln(III) = La, Nd, Eu, Dy, Lu) using 2-thenoyltrifluoroacetone was investigated. Four 1-butyl-3-methylimidazolium bis(perfluoroalkanesulfonyl)imides with different Rf chain length (n = 1-4) were synthesized and used as the extraction solvent. There was no significant difference in the extractability among ILs. The extracted species of each Ln(III) in these IL systems were investigated based on the slope of the plots of the logarithmic distribution ratio of Ln(III) as a function of aqueous phase pH. It was revealed that the extracted complex depends on even or odd number of the Rf chain length n.
Eguchi, Ayano; Okamura, Hiroyuki; Sugita, Tsuyoshi; Ueda, Yuki; Morita, Kotaro*; Shimojo, Kojiro; Naganawa, Hirochika; Hirayama, Naoki*
no journal, ,
In this study, the effect of the perfluoroalkyl (Rf) chain length of the ionic liquid (IL) anion on the IL chelate extraction was investigated. Four 1-butyl-3-methylimidazolium bis(perfluoroalkanesulfonyl)imides with different Rf chain length (n = 1 - 4) were synthesized and used as extraction solvents. Herein, the extraction of trivalent lanthanoids (Ln(III) = La, Nd, Eu, Dy and Lu) with an extractant, 2-thenoyltrifluoroacetone, was studied. It was revealed that the extracted complex depends on even or odd number of the Rf chain length n.
Eguchi, Ayano; Okamura, Hiroyuki; Sugita, Tsuyoshi; Ueda, Yuki; Morita, Kotaro*; Shimojo, Kojiro; Naganawa, Hirochika; Hirayama, Naoki*
no journal, ,
In this study, the effect of the perfluoroalkyl (Rf) chain length of the ionic liquid (IL) anion on the IL chelate extraction of trivalent lanthanoids using 2-thenoyltrifluoroacetone was investigated. Four 1-butyl-3-methylimidazolium bis(perfluoroalkanesulfonyl)imides with different Rf chain length (n = 1-4) were synthesized and used as extraction solvents. The extracted species differed depending on odd-even number of the Rf chain length n, and it was found that the Rf chain length of IL anion has the odd-even effect on the extracted species. The fluorescence lifetime of the extracted Eu(III) complex was measured and the number of coordinating water of Eu(III) was determined. As a result, the Eu(III) complex did not have coordinating water. It was suggested that the Rf chain length of the IL anion does not affect the number of coordinating water of the extracted complex.