Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Kinoshita, Ryoma; Matsumiya, Masahiko*; Shinoku, Kota*; Shiroishi, Hidenobu*
Analytical Sciences, 39(9), p.1575 - 1583, 2023/09
Times Cited Count:0 Percentile:0(Chemistry, Analytical)Extraction of Rh from HCl can be performed by NTAamide(C6) (hexahexyl-nitrilotriacetamide) and other related compounds into n-dodecane. We use ion-pair extraction of anionic species of Rh-chloride and protonated extractant. Rh behave as anion in hydrochloric acid and the tertiary nitrogen atom in extractant may be protonated to produce the quaternary amine in acidic condition. From the present work, the maximum distribution ratio of Rh(III) is 16. The D(Rh) values are changeable during preparation of the aqueous solutions because different Rh-Cl-H O complexes are formed in HCl media and show the slow exchange rate between Cl and H
O complexes are formed in HCl media and show the slow exchange rate between Cl and H O. Using the UV spectrum, Rh-chloride solution having the peak of spectrum at 504 nm can be extracted effectively, where RhCl
O. Using the UV spectrum, Rh-chloride solution having the peak of spectrum at 504 nm can be extracted effectively, where RhCl (H
(H O)
O) and RhCl
and RhCl (H
(H O)
O)
 exist mainly from DFT calculation. Stoichiometry of one-one complex of Rh and NTAamide is obtained from slope analysis, and 85 mM of concentrated Rh ion can be extracted.
exist mainly from DFT calculation. Stoichiometry of one-one complex of Rh and NTAamide is obtained from slope analysis, and 85 mM of concentrated Rh ion can be extracted.
Nomizu, Daiki; Sasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*; Katsuta, Shoichi*
Journal of Radioanalytical and Nuclear Chemistry, 331(3), p.1483 - 1493, 2022/03
Times Cited Count:4 Percentile:68.71(Chemistry, Analytical)We studied the successive formation of water soluble DGA (diglycolamide) and DOODA (dioxaoctanediamide) for the mutual separation of Ln in this extraction system. TODGA (tetraoctyl-diglycolamide) and DOODA(C8) (tetraoctyl-dioxaoctanediamide) have the opposite trend to extract light and heavy Ln through Ln-patterns. Metal-complexes of two folding Ln ions with water-soluble DOODA and three folding with DGA are found and their observed formation constants are calculated. The suitable separation condition (aqueous phase: 30 mM DOODA(C2) in 1 M nitric acid, organic phase: 0.1 M TODGA in n-dodecane) of multi-stage extraction (10  10) is conducted. From the present work, it is clear that La, Pr and Nd are mainly present in aqueous phase, instead Sm-Dy exist in the organic phase.
10) is conducted. From the present work, it is clear that La, Pr and Nd are mainly present in aqueous phase, instead Sm-Dy exist in the organic phase.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Separation Science and Technology, 57(16), p.2543 - 2553, 2022/00
Times Cited Count:3 Percentile:29.84(Chemistry, Multidisciplinary)The mutual separation of actinides (An) from lanthanides (Ln) using the masking agent of DTPA (diethylenetriamine-pentaacetic acid) or DTBA (diethylenetriamine-triacetic acid-bis(diethylacetamide)) in the aqueous phase through DGA extraction, referring TALSPEAK method, is focused. We investigate to obtain the same separation performance using commercially available DTPA on that using DTBA. In this work, we select lactic acid (LA) of pH buffer from 10 organic acids and ethylenediamine (ED) for the pH adjustment. Almost the same D and SF values are obtained among the conditions: TODGA-DTPA-LA-NaOH, TODGA-DTPA-LA-ED, and TODGA-DTBA-LA. The experimental results using batchwise multi-stage extractions show the average yields of Ln (La to Gd) and Am to be 3.73 and 98.1% in the aqueous phase using DGA-DTPA-LA-ED, to be 3.1 and 97.0% using DGA-DTPA-LA-NaOH, and to be 1.61 and 98.7% using DGA-DTBA-LA.
Sasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Solvent Extraction and Ion Exchange, 40(6), p.620 - 640, 2022/00
Times Cited Count:1 Percentile:14.86(Chemistry, Multidisciplinary)Owing to the chemical behavior of trivalent lanthanide and actinide ions with similar ionic radii, realizing this separation is still challenging. All lanthanides, Am, and Cm can be extracted using diglycolamide (DGA), and relatively high An/Ln separation efficiencies have been obtained using diethylenetriamine-triacetic-bisamide (DTBA). To improve the previous results as well as the separation conditions, we used organic acids for pH adjustment. The advantages of this modification included low HNO , DTBA concentrations and pH stability owing to the addition of lactic acid. Under these modified conditions, the recovery rates observed were as follows: 97.1% for Nd with the co-existence of 1.59% Am in organic phase, and 98.4% for Am with the co-existence of 2.95% Nd in aqueous phase.
, DTBA concentrations and pH stability owing to the addition of lactic acid. Under these modified conditions, the recovery rates observed were as follows: 97.1% for Nd with the co-existence of 1.59% Am in organic phase, and 98.4% for Am with the co-existence of 2.95% Nd in aqueous phase.
Kofu, Maiko; Watanuki, Ryuta*; Sakakibara, Toshiro*; Kawamura, Seiko; Nakajima, Kenji; Matsuura, Masato*; Ueki, Takeshi*; Akutsu, Kazuhiro*; Yamamuro, Osamu*
Scientific Reports (Internet), 11(1), p.12098_1 - 12098_8, 2021/06
Times Cited Count:5 Percentile:56.02(Multidisciplinary Sciences)Sasaki, Yuji; Morita, Keisuke; Matsumiya, Masahiko*; Ono, Ryoma*; Shiroishi, Hidenobu*
JOM, 73(4), p.1037 - 1043, 2021/04
Times Cited Count:4 Percentile:34.69(Materials Science, Multidisciplinary)The separation of Dy from Nd is studied from the viewpoint of recycling Dy from Nd magnets. Both metals are lanthanide elements, which means their mutual separation is difficult because of their similar chemical behaviors. All lanthanide elements can be extracted easily by using tetradodecyl-diglycolamide (TDdDGA) extractants, and it has a relatively high separation factor (SF) between Dy and Nd (SF over 10). In the present study, by performing eight extraction steps with the organic phase (0.1M TDdDGA in dodecane), ten steps with an aqueous phase (0.7 M HNO with metals), and six steps with another aqueous phase (0.7 M HNO
with metals), and six steps with another aqueous phase (0.7 M HNO without metals), approximately 99% Dy was recovered into the organic phase with 1% co-extraction of Nd.
without metals), approximately 99% Dy was recovered into the organic phase with 1% co-extraction of Nd.
Matsumiya, Masahiko*; Nomizu, Daiki*; Tsuchida, Yusuke*; Sasaki, Yuji
Hydrometallurgy, 199, p.105539_1 - 105539_8, 2021/02
Times Cited Count:4 Percentile:33.99(Metallurgy & Metallurgical Engineering)The synergistic solvent extraction of lanthanide(III) with mixtures of di-(2-ethylhexyl)phosphoric acid (D2EHPA, A) and monoisodecyl phosphoric acid (MIDPA, B) in phosphonium-based ionic liquid was investigated. In the case of D2EHPA or MIDPA single extractant system, Ln(III) (Ln = Pr and Nd) was extracted as [LnA HA] or [LnB
HA] or [LnB HB], respectively, the extracted species of Tb(III) or Dy(III) were determined by slope analysis. According to the equilibrium constants (
HB], respectively, the extracted species of Tb(III) or Dy(III) were determined by slope analysis. According to the equilibrium constants (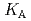 ,
, 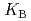 and
and 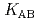 ) and the formation constants (
) and the formation constants (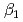 ,
, 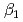 and
and 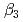 ), it was found that the extracted complex [TbHA
), it was found that the extracted complex [TbHA B
B or [DyHA
or [DyHA B
B ] was more stable than [LnA
] was more stable than [LnA HA] or [LnB
HA] or [LnB HB]. The synergistic extraction effects were investigated to study the possibility of separating Dy(III) from Pr(III) and Nd(III) according to their separation factors.
HB]. The synergistic extraction effects were investigated to study the possibility of separating Dy(III) from Pr(III) and Nd(III) according to their separation factors.
Matsumiya, Masahiko*; Tsuchida, Yusuke*; Sasaki, Yuji; Ono, Ryoma*; Nakase, Masahiko*; Takeshita, Kenji*
Journal of Radioanalytical and Nuclear Chemistry, 327(1), p.597 - 607, 2021/01
Times Cited Count:2 Percentile:24.28(Chemistry, Analytical)To achieve trichotomic separation of light lanthanides (Ln), heavy Ln, and Am, batchwise multi-stage extractions using tetraoctyl-diglycolamide (TODGA) extractant from organic acids are studied. Malonic acid (MA) has high solubility in water and is used as the main component of the aqueous phase. It is clear that the separation factor (SF) for Nd/Am from MA and that for La/Am from MA + HNO are both around 30. The light Ln (e.g., La and Ce) flowed-out in 1 M MA+0.05 M HNO
are both around 30. The light Ln (e.g., La and Ce) flowed-out in 1 M MA+0.05 M HNO (1st soln.), Am is recovered into 3 M MA (2nd soln.), and middle and heavy Ln (Nd and other heavy Ln) are back-extracted into 0.1 M TEDGA/water (3rd soln.). This extraction method can give 95% recovery of Am with total Ln of less than 16% present in high-level radioactive waste.
(1st soln.), Am is recovered into 3 M MA (2nd soln.), and middle and heavy Ln (Nd and other heavy Ln) are back-extracted into 0.1 M TEDGA/water (3rd soln.). This extraction method can give 95% recovery of Am with total Ln of less than 16% present in high-level radioactive waste.
 -diketone extractant and a neutral ligand
-diketone extractant and a neutral ligandMatsumiya, Masahiko*; Nomizu, Daiki*; Tsuchida, Yusuke*; Sasaki, Yuji
Solvent Extraction and Ion Exchange, 39(7), p.764 - 784, 2021/00
Times Cited Count:3 Percentile:22.95(Chemistry, Multidisciplinary)We investigated the solvent extraction of four rare earth (RE) elements (Pr, Nd, Tb, and Dy) from Nd-Fe-B magnets using mixtures of 1-(2-thienyl)-4,4,4,-trifluoro-1,3-butanedione (Htta) or 4,4,4-trifluoro-1-phenyl-1,3-butanedione (Hbfa) chelating extractants and tri-n-octylphosphine oxide (TOPO) neutral ligand in phosphonium based ionic liquids. A synergistic effect was observed for the extraction of the RE elements with the combination of extractant and neutral ligand. The separation of Tb(III) and Dy(III) from other RE(III) components was performed with seven extraction cycles.
Sasaki, Yuji; Matsumiya, Masahiko*; Tsuchida, Yusuke*
Analytical Sciences, 36(11), p.1303 - 1309, 2020/11
Times Cited Count:4 Percentile:16.26(Chemistry, Analytical)The mutual separation of lanthanides is studied by multi-stage extraction using extractant, DGA (diglycolamide) compounds. Tetraoctyl-DGA (TODGA) has a high extractability to lanthanides and relatively high separation factor (SF) between Dy and Nd (SF: over 20). The complete separation with such SF value can be achieved by multi-stage extraction. Less information on multi-stage extraction compared to batch extraction is presented up to now, thus we conduct the basic study about that. Confirming the experimental data to be identical to the calculation, the sample solution including both metals is employed for the batchwise multi-stage extraction. Ninety-seven % of Dy with under detection limit of Nd can be recovered into the organic phase from Nd with ten times higher concentration than Dy using the condition, 0.1 M TODGA/n-dodecane and 0.3 M HNO by multi-stage extraction of 9
by multi-stage extraction of 9 9 for organic and aqueous phases.
9 for organic and aqueous phases.
 -tetraoctyl-diglycolamide from organic acid
-tetraoctyl-diglycolamide from organic acidSasaki, Yuji; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Chemistry Letters, 49(10), p.1216 - 1219, 2020/10
Times Cited Count:9 Percentile:45.29(Chemistry, Multidisciplinary)Lanthanide (Ln) extractions from organic acids to  -dodecane by
-dodecane by  -tetraoctyl-diglycolamide (TODGA) were conducted. Four organic acids (lactic acid, malonic acid, tartaric acid, and citric acid) were employed. Although these acids stabilize lanthanides in the aqueous phase, a distribution ratio (
-tetraoctyl-diglycolamide (TODGA) were conducted. Four organic acids (lactic acid, malonic acid, tartaric acid, and citric acid) were employed. Although these acids stabilize lanthanides in the aqueous phase, a distribution ratio ( ) greater 1 was obtained for heavy Ln. Ln patterns (
) greater 1 was obtained for heavy Ln. Ln patterns ( (Ln) against atomic number of Ln) show maximum values of Ho and Er. In order to obtain high
(Ln) against atomic number of Ln) show maximum values of Ho and Er. In order to obtain high  values, the addition of HNO
values, the addition of HNO in aqueous phase is found to be effective.
in aqueous phase is found to be effective.
 system
systemSasaki, Yuji; Morita, Keisuke; Matsumiya, Masahiko*; Nakase, Masahiko*
Radiochimica Acta, 108(9), p.689 - 699, 2020/09
Times Cited Count:9 Percentile:75.92(Chemistry, Inorganic & Nuclear)The simultaneous separation of Am and Cm from lanthanides is important for atomic energy fields. All lanthanides, Am, and Cm can be extracted by diglycolamide (DGA). In addition, relatively high separation factors between An and Ln were obtained by the extraction system of TODGA, DTPA (diethylenetriamine-pentaacetic acid) and HNO . In this work, DTPA-BA (diethylenetriamine-triacetic-bisamide), which is an improved version of DTPA, was employed for the separation of Ln and An. A relatively high separation factor (approximately 8) for actinides/lanthanides was obtained. Then, the multi-step extraction was performed. Thus, the recoveries of 94.7% for Nd and 4.7% for Am and Cm in organic phase, and 5.3% Nd and 95.3% for Am and Cm in aqueous phase were obtained.
. In this work, DTPA-BA (diethylenetriamine-triacetic-bisamide), which is an improved version of DTPA, was employed for the separation of Ln and An. A relatively high separation factor (approximately 8) for actinides/lanthanides was obtained. Then, the multi-step extraction was performed. Thus, the recoveries of 94.7% for Nd and 4.7% for Am and Cm in organic phase, and 5.3% Nd and 95.3% for Am and Cm in aqueous phase were obtained.
 -dioctylacetamide and direct electrodeposition from loaded organic phase
-dioctylacetamide and direct electrodeposition from loaded organic phaseMatsumiya, Masahiko*; Song, Y.*; Tsuchida, Yusuke*; Sasaki, Yuji
Separation and Purification Technology, 234, p.115841_1 - 115841_8, 2020/03
Times Cited Count:17 Percentile:62.19(Engineering, Chemical)The development of solvent extraction and direct electrodeposition processes is an important task to reduce the volume of secondary wastes. In this study, the extraction of Pd(II) from hydrochloric/chloride media using methylimino-bis- -dioctylacetamide (MIDOA) in three diluents (acetophenone; AP, 1,2-dichloroethane; DCE, or 1-octanol; OC) and the electrochemical behavior of the extracted Pd(II) complex in the MIDOA/AP bath was investigated. Pd(II) was found to be reduced to Pd(0) metal via a two-electron transfer between -2.38 V and -3.40 V. The potentiostatic electrodeposition of the extracted Pd(II) complex enabled us to recover the blackish electrodeposits, which were identified as Pd metal.
-dioctylacetamide (MIDOA) in three diluents (acetophenone; AP, 1,2-dichloroethane; DCE, or 1-octanol; OC) and the electrochemical behavior of the extracted Pd(II) complex in the MIDOA/AP bath was investigated. Pd(II) was found to be reduced to Pd(0) metal via a two-electron transfer between -2.38 V and -3.40 V. The potentiostatic electrodeposition of the extracted Pd(II) complex enabled us to recover the blackish electrodeposits, which were identified as Pd metal.
Sasaki, Yuji; Ban, Yasutoshi; Morita, Keisuke; Matsumiya, Masahiko*; Ono, Ryoma*; Shiroishi, Hidenobu*
Solvent Extraction Research and Development, Japan, 27(1), p.63 - 67, 2020/00
Times Cited Count:6 Percentile:31.74(Chemistry, Multidisciplinary)Mutual separation technique of Dy and Nd in Nd magnet is studied. Dy is more valuable than Nd, then Dy might be isolated and reused. Lanthanide elements can be extracted thoroughly by diglycolamide (DGA) extractants, we use this reagent for the recovery and isolation of Dy. Tetradodecyl-DGA (TDdDGA) has relatively high separation factors(SF) between Dy and Nd (SF=17-18) in HNO extraction system, counter-current extraction using TDdDGA was applied for their mutual separation. From the present study, using the condition, four extraction stages, organic phase: 0.1M TDdDGA in n-dodecane, aqueous phase: 0.3M HNO
extraction system, counter-current extraction using TDdDGA was applied for their mutual separation. From the present study, using the condition, four extraction stages, organic phase: 0.1M TDdDGA in n-dodecane, aqueous phase: 0.3M HNO , 92% Dy can be recovered with 0.7% co-extraction of Nd.
, 92% Dy can be recovered with 0.7% co-extraction of Nd.
Machida, Hideo*; Koizumi, Yu*; Wakai, Takashi; Takahashi, Koji*
Nihon Kikai Gakkai M&M 2019 Zairyo Rikigaku Kanfarensu Koen Rombunshu (Internet), p.OS1307_1 - OS1307_5, 2019/11
This paper describes the fracture test and fracture analysis of a pipe under displacement control load. In order to grasp the fracture behavior of the circumferential through-wall cracked pipe, which is important in evaluating the feasibility of leak before break (LBB) in sodium cooled reactor piping, a fracture test in case of a circumferential throughwall crack in the weld line between an elbow and a straight pipe was carried out. From this test, it was found that no pipe fracture occurs in the displacement control loading condition even if a large circumferential through-wall crack (180 ) was assumed. The fracture analysis of the pipe was carried out using Gurson's parameters set based on the tensile test results of the tested pipe material. The analytic results agree well with the test results, and it was found that it will be possible to predict the fracture behavior of sodium cooled reactor piping.
) was assumed. The fracture analysis of the pipe was carried out using Gurson's parameters set based on the tensile test results of the tested pipe material. The analytic results agree well with the test results, and it was found that it will be possible to predict the fracture behavior of sodium cooled reactor piping.
Sasaki, Yuji; Morita, Keisuke; Matsumiya, Masahiko*; Nakase, Masahiko*
Proceedings of International Nuclear Fuel Cycle Conference / Light Water Reactor Fuel Performance Conference (Global/Top Fuel 2019) (USB Flash Drive), p.108 - 112, 2019/09
We attempted to separate An from Ln, and Am and Cm by the system including extractant and masking agent. The separation factor of Nd and Am was approximately 10 by TODGA-DTPA-BA and that of Am and Cm was over 3 by TODGA-DOODA(C2). Using these batch data, profiles of metal concentration with multi-step extractions proposed in this manuscript were demonstrated.
Sano, Yuichi; Sakamoto, Atsushi; Takeuchi, Masayuki; Misumi, Ryuta*; Kunii, Kanako*; Todoroki, Kei*; Nishi, Kazuhiko*; Kaminoyama, Meguru*
Kagaku Kogaku Rombunshu, 44(6), p.335 - 340, 2018/11
Concerning an annular centrifugal contactor which has high throughput and separation performance, the effect of operational condition on fluidic and dispersion behavior, which are important to improve the contactor performance, was investigated by computational fluid dynamics (CFD) analysis based on the turbulence model, and the calculated results were validated by experimental data. The liquid phase in the annular zone was gradually divided into two regions vertically with increasing the rotor speed and decreasing the flowrate, and the liquid flow moved toward the center of the housing bottom was generated in the lower annular zone under any operational condition. The droplet size of the dispersed phase in the annular zone decreased with increasing the rotor speed and decreasing the flowrate. These calculation results showed a good agreement with experimental data. The CFD analysis considering mass transfer between aqueous and organic phases was also attempted, and it was confirmed that the change of extraction performance with the rotor speed showed the same tendency as the experimental result.
Misumi, Ryuta*; Todoroki, Kei*; Kunii, Kanako*; Nishi, Kazuhiko*; Kaminoyama, Meguru*; Sano, Yuichi; Sakamoto, Atsushi; Takeuchi, Masayuki
Kagaku Kogaku Rombunshu, 44(5), p.285 - 291, 2018/09
Annular centrifugal extractors have been anticipated for use as extractors in spent nuclear fuel recycling. The extraction rate and the liquid-liquid dispersion are related to the flow pattern in the vessel. However, no study has clarified flow patterns in vessels of various scales. For this study, flow pattern characteristics are quantified for extractors of two scales. An extractor has a mixing zone around the vessel bottom and a separation zone in the cylindrical rotor. For this experiment, distilled water was fed into the vessel. Flow behavior in the mixing zone was observed from a side view using a digital video camera at various rotor speeds and supply flow rates for extractors of two scales. In some cases, the liquid horizontal velocity vectors in the mixing zone were measured using particle image velocimetry. Results demonstrate that flow behaviors in the mixing zone in both scales of extractors are classifiable as three types, changing with operational conditions: Type A, Type B, and a Transition regime. For the Type A state, the mixing zone is fully filled with liquid from the vessel bottom up to the lower edge of the rotor. In the Type B state, the zone with existing liquid is vertically divisible into two regions. Lower rotor speeds and higher flow rates tend to produce Type A state flow behavior. The boundary operational condition between Type A and the Transition regime are correlated with the normalized supply flow rate and pumping capacity of the rotor, which is evaluated from liquid surface level in a rotor formed by centrifugal force. Furthermore, the fluid velocity in the mixing zone is roughly proportional to the rotor surface circumferential speed irrespective of the vessel scale.
Nemoto, Fumiya*; Kofu, Maiko; Nagao, Michihiro*; Oishi, Kazuki*; Takata, Shinichi; Suzuki, Junichi*; Yamada, Takeshi*; Shibata, Kaoru; Ueki, Takeshi*; Kitazawa, Yuzo*; et al.
Journal of Chemical Physics, 149(5), p.054502_1 - 054502_11, 2018/08
Times Cited Count:19 Percentile:70.02(Chemistry, Physical)Misumi, Ryuta*; Kunii, Kanako*; Todoroki, Kei*; Nishi, Kazuhiko*; Kaminoyama, Meguru*; Sano, Yuichi; Sakamoto, Atsushi; Takeuchi, Masayuki
Kagaku Kogaku Rombunshu, 44(3), p.135 - 141, 2018/05
Times Cited Count:1 Percentile:4.87(Engineering, Chemical)Annular centrifugal extractors have been used in spent nuclear fuel reprocessing, but the relation between the extraction rate and flow pattern in the vessel remains unclear. This study quantifies characteristics of the flow pattern to clarify this relation. An extractor produces a mixing zone around the vessel bottom and a separation zone in the rotor. The horizontal velocity of the liquid in the mixing zone was measured using particle image velocimetry at various rotor speeds and supply flow rates. Flow behaviors in the mixing zone are of three types, changing with operational conditions: Type A, Type B, and a transition regime. At lower rotor speeds and high supply flow rates, the mixing zone is fully filled with liquid from the vessel bottom up to the lower edge of the rotor: the Type A flow state. At high rotor speeds and low supply flow rates, the zone with existing liquid is vertically divisible into two regions: near the vanes and around the bottom of the rotor, which is the Type B flow state. A transition regime is also observed between Type A and Type B state. In each region surrounding the two vanes on the vessel bottom and the vessel wall, the liquid flowed in the direction of rotor rotation along the vessel wall. Liquid flow altered by the vane flowed toward the center of vessel bottom. The liquid then entered the separation zone through the orifice at the rotor bottom. For the Type A state, the horizontal velocity distribution was roughly proportional to the rotor speed. For the Type B state, the horizontal velocities around the vessel bottom were lower than those of Type A and were not proportional to the rotor speed. Presumably, the liquid fed into the vessel went directly to the rotor instead of passing between the two vanes attached to the vessel bottom.