Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Komuro, Michiyasu; Kanazawa, Hiroyuki; Kokusen, Junya; Shimizu, Osamu; Honda, Junichi; Harada, Katsuya; Otobe, Haruyoshi; Nakada, Masami; Inagawa, Jun
JAEA-Technology 2021-042, 197 Pages, 2022/03
Plutonium Research Building No.1 was constructed in 1960 for the purpose of establishing plutonium handling technology and studying its basic physical properties. Radiochemical research, physicochemical research and analytical chemistry regarding solutions and solid plutonium compounds had been doing for the research program in Japan Atomic Energy Agency (JAEA). In 1964, the laboratory building was expanded and started the researching plutonium-uranium mixed fuel and reprocessing of plutonium-based fuel, playing an advanced role in plutonium-related research in Japan. Since then, the research target has been expanded to include transplutonium elements, and it has functioned as a basic research facility for actinides. The laboratory is constructed by concrete structure and it has the second floor, equipped with 15 glove boxes and 4 chemical hoods. Plutonium Research Building No.1 was decided as one of the facilities to be decommissioned by Japan Atomic Energy Agency Reform Plan in September 2014. So far, the contamination survey of the radioactive materials in the controlled area, the decontamination of glove boxes, and the consideration of the equipment dismantling procedure have been performed as planned. The radioisotope and nuclear fuel materials used in the facility have been transfer to the other facilities in JAEA. The decommissioning of the facility is proceeding with the goal of completing by decommissioning the radiation controlled area in 2026. In this report, the details of the decommissioning plan and the past achievements are reported with the several data.
Inagawa, Jun; Kitatsuji, Yoshihiro; Otobe, Haruyoshi; Nakada, Masami; Takano, Masahide; Akie, Hiroshi; Shimizu, Osamu; Komuro, Michiyasu; Oura, Hirofumi*; Nagai, Isao*; et al.
JAEA-Technology 2021-001, 144 Pages, 2021/08
Plutonium Research Building No.1 (Pu1) was qualified as a facility to decommission, and preparatory operations for decommission were worked by the research groups users and the facility managers of Pu1. The operation of transportation of whole nuclear materials in Pu1 to Back-end Cycle Key Element Research Facility (BECKY) completed at Dec. 2020. In the operation included evaluation of criticality safety for changing permission of the license for use nuclear fuel materials in BECKY, cask of the transportation, the registration request of the cask at the institute, the test transportation, formulation of plan for whole nuclear materials transportation, and the main transportation. This report circumstantially shows all of those process to help prospective decommission.
Kitatsuji, Yoshihiro
Radioisotopes, 67(10), p.483 - 493, 2018/10
Electrochemical reactions and redox properties of actinides such as uranium and neptunium are outlined. The flow electrolysis enables rapid and high-efficient treatment. It was demonstrated to measure slow processes of actinide redox. Experimental results of electrolysis of actinide ions and the preparation method of oxidation state of the ions based on the fundamental data are described. Mediator reaction and catalysis observed in the process of electrolysis of actinide ions are also explained.
Aoyagi, Noboru; Palladino, G.*; Nagasaki, Shinya*; Kimura, Takaumi
Bulletin of the Chemical Society of Japan, 91(6), p.882 - 890, 2018/06
Times Cited Count:1 Percentile:4.18(Chemistry, Multidisciplinary)The speciation and coordination geometries of M(III)-citrate complexes in aqueous solutions, where M denotes Eu, Tb, Lu, or Cm, are studied using potentiometric titration, nuclear magnetic resonance spectroscopies. Their photophysical properties are also characterized by time-resolved fluorescence spectra. The formation constants of mononuclear, dinuclear, and trinuclear Lu-citrate species were determined by potentiometric titration in 3.00 M NaClO aqueous media. Terminal carboxylic conformation in trinuclear complexes comprised both the five- and six-membered rings at different exchanging rates. Hydration states evaluated for Eu
aqueous media. Terminal carboxylic conformation in trinuclear complexes comprised both the five- and six-membered rings at different exchanging rates. Hydration states evaluated for Eu ions are the chemical formula of [Eu(Cit)
ions are the chemical formula of [Eu(Cit) (H
(H O)
O)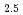 ]
] and [Eu
and [Eu (OH)
(OH) (Cit)
(Cit) (H
(H O)
O)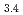 ]
]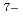 . These complexes in aqueous solution have geometrical similarity to the crystal structures in the literature. Furthermore, the entity of the hetero-trinuclear complex induces the intramolecular energy transfer from Tb
. These complexes in aqueous solution have geometrical similarity to the crystal structures in the literature. Furthermore, the entity of the hetero-trinuclear complex induces the intramolecular energy transfer from Tb to Eu
to Eu . The incorporation of Cm
. The incorporation of Cm into these homo/hetero-trinuclear citrate complexes proved to be a successful trial to probe the formation of actinide polymer at a trace level.
into these homo/hetero-trinuclear citrate complexes proved to be a successful trial to probe the formation of actinide polymer at a trace level.
Segawa, Yukari; Horita, Takuma; Kitatsuji, Yoshihiro; Kumagai, Yuta; Aoyagi, Noboru; Nakada, Masami; Otobe, Haruyoshi; Tamura, Yukito*; Okamoto, Hisato; Otomo, Takashi; et al.
JAEA-Technology 2016-039, 64 Pages, 2017/03
The laboratory building No.1 for the plutonium research program (Bldg. Pu1) was chosen as one of the facilities to decommission by Japan Atomic Energy Agency Reform in September, 2013. The research groups, users of Bldg. Pu1, were driven by necessity to remove used equipment and transport nuclear fuel to other facilities from Bldg. Pu1. Research Group for Radiochemistry proactively established the Used Equipment Removal Team for the smooth operation of the removal in April, 2015. The team classified six types of work into the nature of the operation, removal of used equipment, disposal of chemicals, stabilization of mercury, stabilization of nuclear fuel, transportation of nuclear fuel and radioisotope, and survey of contamination status inside the glove boxes. These works were completed in December, 2015. This report circumstantially shows six works process, with the exception of the approval of the changes on the usage of nuclear fuel in Bldg. Pu1 to help prospective decommission.
Kitatsuji, Yoshihiro
Bunseki, 2015(6), p.239 - 244, 2015/06
Electrochemical studies of ion transfer and charge transfer at liquid/liquid interface reported between 2012 and 2014 were surveyed. They were categorized by method of measurement. The merit of the method, improvement, species of application were described. Applied research such as analyses of redox inactive species, sensitive analyses based on adsorption at the interface and developments of new functional materials had been widely carried out.
 ssbauer spectroscopy of actinide compounds
ssbauer spectroscopy of actinide compoundsTsutsui, Satoshi
M ssbauer Effect Reference and Data Journal, 38(4), p.105 - 111, 2015/06
ssbauer Effect Reference and Data Journal, 38(4), p.105 - 111, 2015/06
M ssbauer spectroscopy of actinide compounds is limited to few facilities in the world. Study of M
ssbauer spectroscopy of actinide compounds is limited to few facilities in the world. Study of M ssbauer spectroscopy of actinides in Japan was started in Advanced Science Research Center in the 1990s. In this paper, we summarize the research results on the M
ssbauer spectroscopy of actinides in Japan was started in Advanced Science Research Center in the 1990s. In this paper, we summarize the research results on the M ssbauer spectroscopy of neptunium intermetallic compound that was started in the Advanced Science Research Center in 1996, and the U-238 M
ssbauer spectroscopy of neptunium intermetallic compound that was started in the Advanced Science Research Center in 1996, and the U-238 M ssbauer spectroscopy started in the institute for Materials Research of Tohoku University. A review of the research results of the M
ssbauer spectroscopy started in the institute for Materials Research of Tohoku University. A review of the research results of the M ssbauer spectroscopy of these actinides is devoted to the late emeritus professor of Osaka University Saburo Nasu, who gives important contributions to its development.
ssbauer spectroscopy of these actinides is devoted to the late emeritus professor of Osaka University Saburo Nasu, who gives important contributions to its development.
Otobe, Haruyoshi; Kitatsuji, Yoshihiro; Kurata, Masaki; Takano, Masahide
Proceedings of 2014 Nuclear Plant Chemistry Conference (NPC 2014) (USB Flash Drive), 11 Pages, 2014/10
The chemical and transport behaviors of Pu and U in the corroded debris must be understood for the criticality safety control of Pu and U in the debris, especially for the removal operations and storage. Therefore, the chemical changes of UO and PuO
and PuO powders, disks and U and Pu metal disks in H
powders, disks and U and Pu metal disks in H O
O aqueous solution have been checked, where H
aqueous solution have been checked, where H O
O is formed by the radiolysis of H
is formed by the radiolysis of H O. As a result, UO
O. As a result, UO changed to hydrated uranium peroxide, whereas the PuO
changed to hydrated uranium peroxide, whereas the PuO remained unchanged. U metal was more reactive with H
remained unchanged. U metal was more reactive with H O
O aqueous solution than Pu metal. The chemical changes of the mixed UO
aqueous solution than Pu metal. The chemical changes of the mixed UO /PuO
/PuO powders in H
powders in H O
O aqueous solution have been investigated. The dried slurry of the middle zone of H
aqueous solution have been investigated. The dried slurry of the middle zone of H O
O aqueous solution was mainly composed of hydrated uranium peroxide, whereas the dried powder of the bottom zone was mainly composed of PuO
aqueous solution was mainly composed of hydrated uranium peroxide, whereas the dried powder of the bottom zone was mainly composed of PuO .
.
Kitatsuji, Yoshihiro; Otobe, Haruyoshi; Kimura, Takaumi; Kihara, Sorin*
Electrochimica Acta, 141, p.6 - 12, 2014/09
Times Cited Count:5 Percentile:9.72(Electrochemistry)Reduction processes of U(VI) in weakly acidic solutions were investigated based on electrochemical and spectrophotometric measurements. A reversible one-electron reduction of U(VI) to U(V) and a further irreversible reduction of U(V) were observed voltammetrically at a gold microdisk electrode in solutions of pH from 2.0 to 3.5. Aggregates of U(IV) were formed as a deposit on the electrode and a colloid in the bulk solution, when the electrolysis was carried out at a gold gauze electrode, even though the potential applied was that available for the first one-electron reduction wave of U(VI) observed. It was elucidated that the aggregate was produced by the combination of the one-electron reduction to U(V) and the disproportionation of U(V) producing U(IV) and U(VI). The aggregate enhanced the rate of the disproportionation of U(V), and hence the reduction current of U(VI) increased abruptly when a definite amount of aggregate was formed on the electrode, in the solution, or both.
 revealed by
revealed by  O-NMR
O-NMRTokunaga, Yo; Nishi, Tsuyoshi; Nakada, Masami; Ito, Akinori*; Sakai, Hironori; Kambe, Shinsaku; Homma, Yoshiya*; Honda, Fuminori*; Aoki, Dai*; Walstedt, R. E.*
Physical Review B, 89(21), p.214416_1 - 214416_8, 2014/06
Times Cited Count:7 Percentile:31.93(Materials Science, Multidisciplinary)The magnetic phase transition near 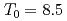 K in AmO
K in AmO has been investigated microscopically by means of
has been investigated microscopically by means of  O NMR. To avoid complexities arising from sample aging associated with the alpha decay of
O NMR. To avoid complexities arising from sample aging associated with the alpha decay of  Am, all measurements have been performed within 40 days after sample synthesis. Even during such a short period, however, a rapid change of NMR line shape has been observed at 1.5 K, suggesting that the ground state of AmO
Am, all measurements have been performed within 40 days after sample synthesis. Even during such a short period, however, a rapid change of NMR line shape has been observed at 1.5 K, suggesting that the ground state of AmO is very sensitive to disorder. We have also confirmed the loss of
is very sensitive to disorder. We have also confirmed the loss of  O NMR signal intensity over a wide temperature range below
O NMR signal intensity over a wide temperature range below 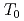 , and more than half of oxygen nuclei are undetectable at 1.5 K. This behavior reveals the persistence of slow and distributed spin fluctuations down to temperatures well below
, and more than half of oxygen nuclei are undetectable at 1.5 K. This behavior reveals the persistence of slow and distributed spin fluctuations down to temperatures well below 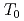 . In the paramagnetic state, strong NMR line broadening and spatially inhomogeneous spin fluctuations have been observed. The results are all indicative of short-range, spin-glass-like character for the magnetic transition in this system.
. In the paramagnetic state, strong NMR line broadening and spatially inhomogeneous spin fluctuations have been observed. The results are all indicative of short-range, spin-glass-like character for the magnetic transition in this system.
Kitatsuji, Yoshihiro
Review of Polarography, 60(1), p.25 - 34, 2014/05
no abstracts in English
Kimura, Takaumi
Hosha Kagaku, (29), p.26 - 33, 2014/04
no abstracts in English
Otobe, Haruyoshi
Journal of Nuclear Materials, 442(1-3), p.394 - 399, 2013/11
Times Cited Count:1 Percentile:10.69(Materials Science, Multidisciplinary)The chemical properties (ex. atomic diffusion), the thermal properties (ex. melting point and thermal diffusivity) and the mechanical properties of oxide fuels are significantly dependent on the oxygen defects of the oxide fuels. For the first step to specify the oxygen defects of oxide fuels, we have tried the thermodynamic approaches to the oxygen defects by comparing the relations between the molar ratio of oxygen to metal (O/M) and the oxygen potentials of Pu, Am, Cm, Ce oxides and several solid solutions including Zr and Np dioxides. Consequently, we have found the consistent properties in the relations between O/M and the oxygen potentials of actinide, lanthanide oxides and their solid solutions, in addition to the dependence of the relations on the crystal structures.
 ssbauer and magnetic study of neptunyl(+1) complexes
ssbauer and magnetic study of neptunyl(+1) complexesNakamoto, Tadahiro*; Nakamura, Akio; Takeda, Masuo*
M ssbauer Spectroscopy; Applications in Chemistry, Biology, and Nanotechnology, p.95 - 114, 2013/10
ssbauer Spectroscopy; Applications in Chemistry, Biology, and Nanotechnology, p.95 - 114, 2013/10
 U M
U M ssbauer spectroscopy
ssbauer spectroscopyTsutsui, Satoshi; Nakada, Masami
M ssbauer Spectroscopy; Applications in Chemistry, Biology, and Nanotechnology, p.123 - 140, 2013/10
ssbauer Spectroscopy; Applications in Chemistry, Biology, and Nanotechnology, p.123 - 140, 2013/10
no abstracts in English
Kitatsuji, Yoshihiro; Kimura, Takaumi; Kihara, Sorin*
Electrochimica Acta, 74, p.215 - 221, 2012/07
Times Cited Count:2 Percentile:5.39(Electrochemistry)Redox reactions of U, Np and Pu ions of various oxidation states were investigated by flow electrolysis at a column electrode (CE) equipped with a platinized glassy carbon (GC) fiber working electrode (Pt/GC-WE), and compared with those observed at the CE with an ordinary activated GC fiber working electrode (GC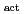 -WE). Since the overpotential for the reduction of NpO
-WE). Since the overpotential for the reduction of NpO
 and PuO
and PuO
 were decreased when Pt/GC-WE was employed, the one-electron reduction of NpO
were decreased when Pt/GC-WE was employed, the one-electron reduction of NpO
 to Np
to Np followed by that of Np
followed by that of Np to Np
to Np and the one-step three-electron reduction of PuO
and the one-step three-electron reduction of PuO
 to Pu
to Pu proceeded at the CE with Pt/GC-WE, different from the reduction processes of NpO
proceeded at the CE with Pt/GC-WE, different from the reduction processes of NpO
 and PuO
and PuO
 at the CE with GC
at the CE with GC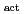 -WE. A rapid and precise method for the preparation of U, Np and Pu ion of a desired oxidation state was proposed by taking advantage of the unique characteristics of the CE with Pt/GC-WE.
-WE. A rapid and precise method for the preparation of U, Np and Pu ion of a desired oxidation state was proposed by taking advantage of the unique characteristics of the CE with Pt/GC-WE.
Sasaki, Takayuki*; Yoshida, Hatsumi*; Kitatsuji, Yoshihiro; Takagi, Ikuji*; Moriyama, Hirotake*
Chemistry Letters, 40(8), p.870 - 871, 2011/08
Times Cited Count:1 Percentile:6.74(Chemistry, Multidisciplinary)Trivalent metal ion selective electrode (ISE) consisting of bis-(diphenylphosphoryl)-methane as an ionophore was developed for the determination of formation constants with organic ligands. The ISE prepared exhibited Nernstian response to the concentration of Eu in the test solutions. Even in the presence of carboxylic acids such as malonic acid in samples, the obtained potential values were found to be stable and reproducible during the measurement time. The present formation constants of Eu with carboxylates were in good agreement with the reported values.
in the test solutions. Even in the presence of carboxylic acids such as malonic acid in samples, the obtained potential values were found to be stable and reproducible during the measurement time. The present formation constants of Eu with carboxylates were in good agreement with the reported values.
Kitatsuji, Yoshihiro; Okugaki, Tomohiko*; Kasuno, Megumi*; Kubota, Hiroki*; Maeda, Koji*; Kimura, Takaumi; Yoshida, Zenko; Kihara, Sorin*
Journal of Chemical Thermodynamics, 43(6), p.844 - 851, 2011/06
Times Cited Count:8 Percentile:30.28(Thermodynamics)Standard Gibbs energies for transfer ( G
G
 ) of actinyl ions (AnO
) of actinyl ions (AnO
 ; z = 2 or 1; An: U, Np or Pu) between an aqueous solution and an organic solution were determined based on distribution method combined with voltammetry for ion transfer at the interface of two immiscible electrolyte solutions. The organic solutions examined were nitrobenzene, 1,2-dichloroethane, benzonitrile, acetophenone and 2-nitrophenyl octyl ether. Irrespective of the type of organic solutions,
; z = 2 or 1; An: U, Np or Pu) between an aqueous solution and an organic solution were determined based on distribution method combined with voltammetry for ion transfer at the interface of two immiscible electrolyte solutions. The organic solutions examined were nitrobenzene, 1,2-dichloroethane, benzonitrile, acetophenone and 2-nitrophenyl octyl ether. Irrespective of the type of organic solutions,  G
G
 of UO
of UO
 , NpO
, NpO
 and PuO
and PuO
 were nearly equal to each other and slightly larger than that of Mg
were nearly equal to each other and slightly larger than that of Mg . The
. The  G
G
 of NpO
of NpO
 was extraordinary large compared with those of ordinary monovalent cations. The dependence of
was extraordinary large compared with those of ordinary monovalent cations. The dependence of  G
G
 of AnO
of AnO
 on the type of organic solutions was similar to that of H
on the type of organic solutions was similar to that of H or Mg
or Mg . The
. The  G
G
 of An
of An and An
and An were also discussed briefly.
were also discussed briefly.
Kitatsuji, Yoshihiro; Kimura, Takaumi; Kihara, Sorin*
IOP Conference Series; Materials Science and Engineering, 9, p.012078_1 - 012078_7, 2010/05
Times Cited Count:0 Percentile:1.02(Chemistry, Inorganic & Nuclear)The behaviors of reduction of Np(V) by controlled potential electrolysis were studied, and a unique time course of electrolysis current was observed. It was conclude that Np(V) was reduced by two reduction processes that are the chemical reaction with Np(III) and the electrocatalytic reduction by adsorbed hydrogen atom on platinum electrode surface. The time course of current for controlled potential electrolysis of Np(V) under various condition of the solution was investigated, and the effects of the concentration of H and NO
and NO
 on electrolysis behaviour were shown.
on electrolysis behaviour were shown.
Kitatsuji, Yoshihiro; Kimura, Takaumi; Kihara, Sorin*
Journal of Electroanalytical Chemistry, 641(1-2), p.83 - 89, 2010/03
Unique current-time relations were observed during the controlled potential electrolysis for the reduction of Np(V) to Np(IV) or Np(III) at a Au or Pt electrode. The reduction process of Np(V) was elucidated based on bulk electrolysis and voltammetry: Though Np(V) is not reduced directly at a Au electrode, it is reduced by chemical reaction with Np(III) which is produced by the electrode reduction of Np(IV). The reduction of Np(V) at a Pt electrode is initiated by the electrocatalytic reduction to Np(IV) with the hydrogen atom which is produced by the electrode reduction of H and adsorbed on the Pt electrode surface. The Np(IV) produced functions as an electron transfer mediator to reduce Np(V) similarly to the reaction at a Au electrode. The above described catalytic reduction processes of Np(V) were elucidated with the aid of digital simulation. Methods useful for the preparation of Np(IV) and Np(III) by bulk electrolysis were proposed based on the experimental results.
and adsorbed on the Pt electrode surface. The Np(IV) produced functions as an electron transfer mediator to reduce Np(V) similarly to the reaction at a Au electrode. The above described catalytic reduction processes of Np(V) were elucidated with the aid of digital simulation. Methods useful for the preparation of Np(IV) and Np(III) by bulk electrolysis were proposed based on the experimental results.