Sorption and Diffusion Behavior of Palladium in Bentonite, Granodiorite and Tuff
not registered 
 ; not registered; Sato, Haruo; Shibata, Masahiro
; not registered; Sato, Haruo; Shibata, Masahiro 
Sorption and diffusion behavior of palladium, which has been identified as one of the hazardous radionuclides in performance assessment of HLW disposal, in bentonite, granodiorite and tuff was studied in order to make reliable data set for the performance assessment. Sorption experiments of Pd on bentonite, granodiorite and tuff were conducted as functions of pH, ionic strength and liquid to solid ratio by batch method under aerobic conditions at room temperature. The distribution coefficients (K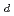 ) of Pd on these solids were almost in the range of 10
) of Pd on these solids were almost in the range of 10 to 10
to 10 m
m /kg and were in the order of bentonite
/kg and were in the order of bentonite  granodiorite
granodiorite 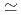 tuff. The sorption trends with change in PH, ionic strength and liquid to solid ratio are very similar between three solids. The K
tuff. The sorption trends with change in PH, ionic strength and liquid to solid ratio are very similar between three solids. The K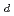 values were the highest at pH5 and decreased with increasing pH between 5 and 11. The effect of ionic strength on K
values were the highest at pH5 and decreased with increasing pH between 5 and 11. The effect of ionic strength on K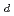 was not found in a range of 10
was not found in a range of 10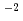 to 10
to 10 , but K
, but K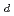 values increased with increasing liquid to solid ratio. The width of variation in K
values increased with increasing liquid to solid ratio. The width of variation in K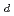 was one order of magnitude in a liquid to solid ratio of 0.1 to 1 m
was one order of magnitude in a liquid to solid ratio of 0.1 to 1 m /kg. Sorption behavior of Pd is different from that of divalent metal ions such as Ni and Co etc. and chemical analogy may be inappropriate. The dominant aqueous species of Pd in the expermental conditions studied is estimated to be neutral species, Pd(OH)
/kg. Sorption behavior of Pd is different from that of divalent metal ions such as Ni and Co etc. and chemical analogy may be inappropriate. The dominant aqueous species of Pd in the expermental conditions studied is estimated to be neutral species, Pd(OH) (aq) by the thermodynamic calculations. The K
(aq) by the thermodynamic calculations. The K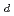 values of Pd on three solids were relatively high and uncharged complexes may be more strongly sorbed. The pH dependency of K
values of Pd on three solids were relatively high and uncharged complexes may be more strongly sorbed. The pH dependency of K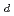 values suggests that Pd sorption is most likely to be occurring onto positively charged S-OH
values suggests that Pd sorption is most likely to be occurring onto positively charged S-OH type site which are progressively removed (to from SOH and SO
type site which are progressively removed (to from SOH and SO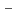 sites) at higher pH values. Diffusion behavior of Pd in bentonite was also studied by in-diffusion method as a function of dry density. The D
sites) at higher pH values. Diffusion behavior of Pd in bentonite was also studied by in-diffusion method as a function of dry density. The D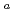 values obtained based on the instantaneous planar source model were in the orders of ...
values obtained based on the instantaneous planar source model were in the orders of ...