Sorption behavior of neptunium onto smectite under reducing conditions in carbonate media
Kitamura, Akira 
 ; Tomura, Tsutomu*; not registered
; Tomura, Tsutomu*; not registered
Sorption behavior of neptunium onto smectite was investigated upder reducing conditions in carbonate media. Distribution coefficient (K ) of neptunium on smectite was determined by a batch method as a function of total carbonate concentration (C
) of neptunium on smectite was determined by a batch method as a function of total carbonate concentration (C ) in a weak alkaline solution. The C
) in a weak alkaline solution. The C value ranged from 0.09 M (M
value ranged from 0.09 M (M mol
mol dm
dm ) to 1.0 M. The obtained K
) to 1.0 M. The obtained K value decreased with increase the total carbonate concentration. It was found that both Np(IV) and Np(V) coexisted in the solution by a TTA-extraction method. In the present experimental conditions, contribution of neptunyl ion, NpO
value decreased with increase the total carbonate concentration. It was found that both Np(IV) and Np(V) coexisted in the solution by a TTA-extraction method. In the present experimental conditions, contribution of neptunyl ion, NpO
 , carbonate complexes of Np(V), NpO
, carbonate complexes of Np(V), NpO (CO
(CO )
)
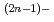 (n=1, 2 and 3), and a carbonatohydroxo complex of Np(IV), Np(CO
(n=1, 2 and 3), and a carbonatohydroxo complex of Np(IV), Np(CO )
) (OH)
(OH)
 , to the sorption onto smectite was expected. The K
, to the sorption onto smectite was expected. The K values of respective species of neptunium were estimated by a least-squares fit to the experimental data. From the obtained K
values of respective species of neptunium were estimated by a least-squares fit to the experimental data. From the obtained K values of respective species, sorption behavior of neptunium onto smectite was discussed.
values of respective species, sorption behavior of neptunium onto smectite was discussed.