Oxygen Potentials of (U,Pu,Am)O ; Measurement and Modeling
; Measurement and Modeling
Osaka, Masahiko 
 ; Sato, Isamu; Namekawa, Takashi; Tanaka, Kenya; Ishida, Takashi*
; Sato, Isamu; Namekawa, Takashi; Tanaka, Kenya; Ishida, Takashi*
Oxygen potentials of a nonstoichiometric oxide, (U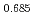 Pu
Pu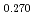 Am
Am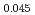 )O
)O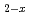 , were measured as a function of oxygen to metal (O/M) ratio at 1123 K, 1273 K and 1423 K by thermogravimetric analysis (TGA) with CO
, were measured as a function of oxygen to metal (O/M) ratio at 1123 K, 1273 K and 1423 K by thermogravimetric analysis (TGA) with CO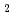 /H
/H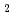 and H
and H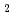 O/H
O/H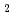 gas equilibria. The oxygen potentials of (U
gas equilibria. The oxygen potentials of (U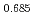 Pu
Pu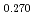 Am
Am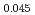 )O
)O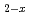 were significantly higher than those of MOX containing no Am, although the content of Am is only 4.5%. The defect structure was examined from slopes of the oxygen partial pressure versus deviation from stoichiometry. A high slope value was observed in (U
were significantly higher than those of MOX containing no Am, although the content of Am is only 4.5%. The defect structure was examined from slopes of the oxygen partial pressure versus deviation from stoichiometry. A high slope value was observed in (U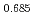 Pu
Pu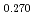 Am
Am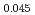 )O
)O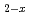 , which is attributed not to defects or defect clusters, but to possible formation of an ordered intermediate phase. This fact was supported by the shape of oxygen partial molar enthalpy and entropy. The oxygen potential isotherms of (U,Pu,Am)O
, which is attributed not to defects or defect clusters, but to possible formation of an ordered intermediate phase. This fact was supported by the shape of oxygen partial molar enthalpy and entropy. The oxygen potential isotherms of (U,Pu,Am)O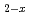 were represented by a chemical thermodynamic model proposed by Lindemer et al. It was assumed in the present model that (U,Pu,Am)O
were represented by a chemical thermodynamic model proposed by Lindemer et al. It was assumed in the present model that (U,Pu,Am)O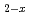 consisted of the five chemical species in a pseudo-quaternary system by treating the reduction rates of Pu and Am as identical; furthermore an interaction between and was introduced. The agreement between analytical and experimental isotherms was good.
consisted of the five chemical species in a pseudo-quaternary system by treating the reduction rates of Pu and Am as identical; furthermore an interaction between and was introduced. The agreement between analytical and experimental isotherms was good.