(U,Pu,Am)O の酸素ポテンシャル; 測定及び解析モデルの構築
の酸素ポテンシャル; 測定及び解析モデルの構築
Oxygen Potentials of (U,Pu,Am)O ; Measurement and Modeling
; Measurement and Modeling
逢坂 正彦 
 ; 佐藤 勇; 滑川 卓志; 田中 健哉; 石田 貴志*
; 佐藤 勇; 滑川 卓志; 田中 健哉; 石田 貴志*
Osaka, Masahiko; Sato, Isamu; Namekawa, Takashi; Tanaka, Kenya; Ishida, Takashi*
不定比組成を有する(U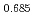 Pu
Pu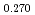 Am
Am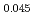 )O
)O の酸素ポテンシャルを、CO
の酸素ポテンシャルを、CO /H
/H 及びH
及びH O/H
O/H 気相平衡を用いた熱重量分析法により、O/M比の関数として、1123K, 1273K及び1423 Kにおいて実験的に求めた。その結果、僅か4.5 %のAmであっても、(U,Pu)O
気相平衡を用いた熱重量分析法により、O/M比の関数として、1123K, 1273K及び1423 Kにおいて実験的に求めた。その結果、僅か4.5 %のAmであっても、(U,Pu)O へ添加することにより、その酸素ポテンシャルを大幅に上昇させることがわかった。(U
へ添加することにより、その酸素ポテンシャルを大幅に上昇させることがわかった。(U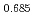 Pu
Pu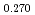 Am
Am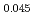 )O
)O の欠陥構造を、酸素分圧と定比からのずれの傾きから考察した。その結果、O/M=1.990と1.970の間に見られた大きな傾きは、欠陥が規則化した中間相の形成によるものである可能性が示唆された。これは酸素の部分モルエンタルピー及びエントロピーのO/M比依存性からも裏付けられた。実際の高速炉での照射挙動評価に資するために、化学熱力学モデルを用いて酸素ポテンシャル曲線の解析モデルを構築した。化学種として5つを考慮し、AmとPuの還元比率を一定とした。(U
の欠陥構造を、酸素分圧と定比からのずれの傾きから考察した。その結果、O/M=1.990と1.970の間に見られた大きな傾きは、欠陥が規則化した中間相の形成によるものである可能性が示唆された。これは酸素の部分モルエンタルピー及びエントロピーのO/M比依存性からも裏付けられた。実際の高速炉での照射挙動評価に資するために、化学熱力学モデルを用いて酸素ポテンシャル曲線の解析モデルを構築した。化学種として5つを考慮し、AmとPuの還元比率を一定とした。(U Am
Am )O
)O 酸素ポテンシャルの解析から 相互作用を求めて新たに導入することにより、実験値を良好に再現する曲線を得た。
酸素ポテンシャルの解析から 相互作用を求めて新たに導入することにより、実験値を良好に再現する曲線を得た。
Oxygen potentials of a nonstoichiometric oxide, (U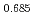 Pu
Pu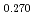 Am
Am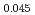 )O
)O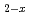 , were measured as a function of oxygen to metal (O/M) ratio at 1123 K, 1273 K and 1423 K by thermogravimetric analysis (TGA) with CO
, were measured as a function of oxygen to metal (O/M) ratio at 1123 K, 1273 K and 1423 K by thermogravimetric analysis (TGA) with CO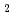 /H
/H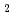 and H
and H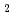 O/H
O/H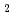 gas equilibria. The oxygen potentials of (U
gas equilibria. The oxygen potentials of (U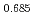 Pu
Pu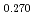 Am
Am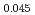 )O
)O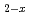 were significantly higher than those of MOX containing no Am, although the content of Am is only 4.5%. The defect structure was examined from slopes of the oxygen partial pressure versus deviation from stoichiometry. A high slope value was observed in (U
were significantly higher than those of MOX containing no Am, although the content of Am is only 4.5%. The defect structure was examined from slopes of the oxygen partial pressure versus deviation from stoichiometry. A high slope value was observed in (U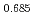 Pu
Pu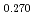 Am
Am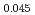 )O
)O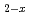 , which is attributed not to defects or defect clusters, but to possible formation of an ordered intermediate phase. This fact was supported by the shape of oxygen partial molar enthalpy and entropy. The oxygen potential isotherms of (U,Pu,Am)O
, which is attributed not to defects or defect clusters, but to possible formation of an ordered intermediate phase. This fact was supported by the shape of oxygen partial molar enthalpy and entropy. The oxygen potential isotherms of (U,Pu,Am)O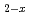 were represented by a chemical thermodynamic model proposed by Lindemer et al. It was assumed in the present model that (U,Pu,Am)O
were represented by a chemical thermodynamic model proposed by Lindemer et al. It was assumed in the present model that (U,Pu,Am)O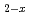 consisted of the five chemical species in a pseudo-quaternary system by treating the reduction rates of Pu and Am as identical; furthermore an interaction between and was introduced. The agreement between analytical and experimental isotherms was good.
consisted of the five chemical species in a pseudo-quaternary system by treating the reduction rates of Pu and Am as identical; furthermore an interaction between and was introduced. The agreement between analytical and experimental isotherms was good.