 Raman spectroscopic observation of corrosion reaction of Fe with Na
Raman spectroscopic observation of corrosion reaction of Fe with Na O
O up to 833 K
up to 833 K
Otori, Norikazu; Furukawa, Tomohiro*; Ueno, Fumiyoshi 

Raman spectra have been obtained for alfa- and beta-NaFeO , Na
, Na FeO
FeO , Na
, Na Fe
Fe O
O , Na
, Na FeO
FeO , and Na
, and Na FeO
FeO from room temperature up to 723 K in a state of powder under an inert atmosphere. The comparison of the spectra showed good applicability of Raman spectroscopy to the in situ identification between these sodium iron double oxides. On the basis of this result, we have investigated corrosion reaction on surface of steel with Na
from room temperature up to 723 K in a state of powder under an inert atmosphere. The comparison of the spectra showed good applicability of Raman spectroscopy to the in situ identification between these sodium iron double oxides. On the basis of this result, we have investigated corrosion reaction on surface of steel with Na O
O powder using in situ Raman spectroscopy. The obtained spectra showed corrosion reaction occurs under 723 K and the corrosion product was identified as Na5FeO4. A reaction mechanism for the corrosion based on the above results was presented that the system of Fe + Na2O2 produces corrosive melt under 723 K so that it spreads over the surface and the corrosion products distribute homogeneously on the surface. The corrosion reaction in homogeneous powder mixture of Fe and Na
powder using in situ Raman spectroscopy. The obtained spectra showed corrosion reaction occurs under 723 K and the corrosion product was identified as Na5FeO4. A reaction mechanism for the corrosion based on the above results was presented that the system of Fe + Na2O2 produces corrosive melt under 723 K so that it spreads over the surface and the corrosion products distribute homogeneously on the surface. The corrosion reaction in homogeneous powder mixture of Fe and Na O
O has also been investigated using Raman spectroscopy with the help of XRD and DTA methods. The corrosion products were identified as the double oxides whose compositions coorespond to the stoichiometric ratio of Na to Fe in the starting materials, while the products from the surface reaction of steel with Na
has also been investigated using Raman spectroscopy with the help of XRD and DTA methods. The corrosion products were identified as the double oxides whose compositions coorespond to the stoichiometric ratio of Na to Fe in the starting materials, while the products from the surface reaction of steel with Na O
O tend to have a spcified composition of Na
tend to have a spcified composition of Na FeO
FeO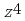 . The differnce can reasonably be explained using the mechanism presented above. It was found from the DTA measurements that Na
. The differnce can reasonably be explained using the mechanism presented above. It was found from the DTA measurements that Na O
O has particularly strong corrosivity for iron, in contrast to Na
has particularly strong corrosivity for iron, in contrast to Na O and NaOH.
O and NaOH.