 Raman spectroscopic observation of corrosion reaction of Fe with Na
Raman spectroscopic observation of corrosion reaction of Fe with Na O
O up to 833 K
up to 833 K
None
大鳥 範和; 古川 智弘*; 上野 文義 

Otori, Norikazu; Furukawa, Tomohiro*; Ueno, Fumiyoshi
 及び
及び .NaFeO
.NaFeO 、Na
、Na FeO
FeO 、Na
、Na Fe
Fe O
O 、Na
、Na FeO
FeO 、およびNa
、およびNa FeO
FeO について粉末状態で室温から723Kまでの温度領域でラマン散乱測定を行った。その結果、それぞれの複合酸化物が主成分である場合、ラマン分光法がこれらの個々の物質の同定法として有用であることがわかった。この結果を基に、Na
について粉末状態で室温から723Kまでの温度領域でラマン散乱測定を行った。その結果、それぞれの複合酸化物が主成分である場合、ラマン分光法がこれらの個々の物質の同定法として有用であることがわかった。この結果を基に、Na O
O による鉄平底セル底部表面の界面腐食反応をラマン分光法で調べた結果、Na
による鉄平底セル底部表面の界面腐食反応をラマン分光法で調べた結果、Na O
O がFeと接する界面系における723Kまでの腐食生成物はN
がFeと接する界面系における723Kまでの腐食生成物はN a5FeO
a5FeO と同定された。上記の結果がセル形状に依存せず、鉄表面全体にNa
と同定された。上記の結果がセル形状に依存せず、鉄表面全体にNa FeO
FeO が均一に生成していること、またNa
が均一に生成していること、またNa O
O に直接接触していないはずの鉄表面でもNa
に直接接触していないはずの鉄表面でもNa FeO
FeO が生成する実に基づいて、Na
が生成する実に基づいて、Na O
O がFeと接する界面系では、723KまでのNa
がFeと接する界面系では、723KまでのNa FeO
FeO を生成する腐食の初期反応の途中において不純物のNaOHによって一旦融体を生成し、金属表面を液体状態で拡散するという腐食のメカニズムが提示された。このメカニズムは、Na
を生成する腐食の初期反応の途中において不純物のNaOHによって一旦融体を生成し、金属表面を液体状態で拡散するという腐食のメカニズムが提示された。このメカニズムは、Na O
O とFeそれぞれ粉末の均一混合物の腐食挙動についても適切に説明できることがわかった。DTA測定の結果から、573K以上でNa
とFeそれぞれ粉末の均一混合物の腐食挙動についても適切に説明できることがわかった。DTA測定の結果から、573K以上でNa O
O がNa
がNa OやNaOHと比べ、鉄に対して特異的に強い腐食性を有することが明らかになった。
OやNaOHと比べ、鉄に対して特異的に強い腐食性を有することが明らかになった。
Raman spectra have been obtained for alfa- and beta-NaFeO , Na
, Na FeO
FeO , Na
, Na Fe
Fe O
O , Na
, Na FeO
FeO , and Na
, and Na FeO
FeO from room temperature up to 723 K in a state of powder under an inert atmosphere. The comparison of the spectra showed good applicability of Raman spectroscopy to the in situ identification between these sodium iron double oxides. On the basis of this result, we have investigated corrosion reaction on surface of steel with Na
from room temperature up to 723 K in a state of powder under an inert atmosphere. The comparison of the spectra showed good applicability of Raman spectroscopy to the in situ identification between these sodium iron double oxides. On the basis of this result, we have investigated corrosion reaction on surface of steel with Na O
O powder using in situ Raman spectroscopy. The obtained spectra showed corrosion reaction occurs under 723 K and the corrosion product was identified as Na5FeO4. A reaction mechanism for the corrosion based on the above results was presented that the system of Fe + Na2O2 produces corrosive melt under 723 K so that it spreads over the surface and the corrosion products distribute homogeneously on the surface. The corrosion reaction in homogeneous powder mixture of Fe and Na
powder using in situ Raman spectroscopy. The obtained spectra showed corrosion reaction occurs under 723 K and the corrosion product was identified as Na5FeO4. A reaction mechanism for the corrosion based on the above results was presented that the system of Fe + Na2O2 produces corrosive melt under 723 K so that it spreads over the surface and the corrosion products distribute homogeneously on the surface. The corrosion reaction in homogeneous powder mixture of Fe and Na O
O has also been investigated using Raman spectroscopy with the help of XRD and DTA methods. The corrosion products were identified as the double oxides whose compositions coorespond to the stoichiometric ratio of Na to Fe in the starting materials, while the products from the surface reaction of steel with Na
has also been investigated using Raman spectroscopy with the help of XRD and DTA methods. The corrosion products were identified as the double oxides whose compositions coorespond to the stoichiometric ratio of Na to Fe in the starting materials, while the products from the surface reaction of steel with Na O
O tend to have a spcified composition of Na
tend to have a spcified composition of Na FeO
FeO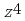 . The differnce can reasonably be explained using the mechanism presented above. It was found from the DTA measurements that Na
. The differnce can reasonably be explained using the mechanism presented above. It was found from the DTA measurements that Na O
O has particularly strong corrosivity for iron, in contrast to Na
has particularly strong corrosivity for iron, in contrast to Na O and NaOH.
O and NaOH.