Mechanism of radiation-induced reactions in aqueous solution of coumarin-3-carboxylic acid; Effects of concentration, gas and additive on fluorescent product yield
Yamashita, Shinichi; Baldacchino, G.*; Maeyama, Takuya*; Taguchi, Mitsumasa; Muroya, Yusa*; Lin, M.*; Kimura, Atsushi; Murakami, Takeshi*; Katsumura, Yosuke
Radiation-induced reactions in aqueous solutions of a water-soluble coumarin derivative, coumarin-3-carboxyl acid (C3CA), have been investigated by pulse radiolysis with 35-MeV electron beam, final product analysis after  Co
Co 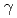 -irradiations, and deterministic model simulations. It was found that C3CA reacts with the hydroxyl radical (
-irradiations, and deterministic model simulations. It was found that C3CA reacts with the hydroxyl radical ( OH) as well as the hydrated electron at nearly diffusion-controlled rate constants: 6.8
OH) as well as the hydrated electron at nearly diffusion-controlled rate constants: 6.8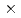 10
10 and 2.1
and 2.1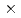 10
10 M
M s
s , respectively. Reactivity of C3CA toward O
, respectively. Reactivity of C3CA toward O

 was not confirmed. Production of a fluorescent molecule 7-hydroxy-coumarin-3-carboxylic acid (7OH-C3CA) was detected by a fluorescence spectrometer coupled with high performance liquid chromatography. Production yields of 7OH-C3CA were in a range from 0.025 to 0.18 (100 eV)
was not confirmed. Production of a fluorescent molecule 7-hydroxy-coumarin-3-carboxylic acid (7OH-C3CA) was detected by a fluorescence spectrometer coupled with high performance liquid chromatography. Production yields of 7OH-C3CA were in a range from 0.025 to 0.18 (100 eV) , depending on irradiation conditions. A variety of the yield with saturating gas, additive, and C3CA concentration implied that there are at least two pathways from scavenging reaction of C3CA toward
, depending on irradiation conditions. A variety of the yield with saturating gas, additive, and C3CA concentration implied that there are at least two pathways from scavenging reaction of C3CA toward  OH to 7OH-C3CA: peroxidation reaction followed by elimination of perhydroxyl radical and disproportionation reaction. A reaction mechanism involving the two pathways was proposed and incorporated into the simulations, showing good explanation of experimentally measured 7OH-C3CA yields with a constant conversion factor from
OH to 7OH-C3CA: peroxidation reaction followed by elimination of perhydroxyl radical and disproportionation reaction. A reaction mechanism involving the two pathways was proposed and incorporated into the simulations, showing good explanation of experimentally measured 7OH-C3CA yields with a constant conversion factor from  OH scavenging to 7OH-C3CA production, 4.7%, unless
OH scavenging to 7OH-C3CA production, 4.7%, unless 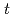 -BuOH is not added.
-BuOH is not added.