突然変異抑制酵素NUDT5の幅広い基質特異性発現機構
Mechanistic insight into hydrolysis of oxidized nucleotide diphosphates by human NUDT5
有森 貴夫; 山縣 ゆり子*
Arimori, Takao; Yamagata, Yuriko*
DNAは、さまざまな損傷を受けることが知られており、生物はこれらの損傷に対する多様な防御機構を備えている。DNAの塩基部位の酸化は代表的な損傷の一つである。DNAを構成する塩基の中でも、グアニンは最も酸化損傷を受けやすく、その中でも最も生成されやすい8-オキソグアニン(8oxoG)は、シトシンだけでなくアデニンともミス塩基対を形成してしまうため、DNAのトランスバージョン変異を引き起こす要因となる。ヒトNUDT5は、8oxoGなどを含む多くの酸化損傷ヌクレオチドとADP-リボースをはじめとする複数のADP-sugarを加水分解することから、幅広く細胞内の浄化に寄与している酵素といえる。最近われわれは、NUDT5と酸化ヌクレオチドとの複合体の立体構造解析の結果などから、NUDT5が酵素学的にも大変興味深い非常にユニークな機構によりさまざまな基質を認識し、加水分解していることを明らかにした。本稿では、その立体構造解析の結果を中心に、NUDT5による基質認識機構及び加水分解反応機構を概説する。
Human NUDT5 hydrolyzes 8-oxo-dGDP into 8-oxo-dGMP and inorganic phosphate and prevents mutations caused by misincorporation of 8-oxoG into DNA. In addition, NUDT5 displays a broad substrate specificity for various modified nucleotides including 8-oxo-dADP, 8-oxo-GDP, and ADP-ribose. To understand the mechanisms of substrate recognition and catalysis by NUDT5, we have determined the crystal structure of NUDT5 complexed with 8-oxo-dGDP. A comparison of the structure with the previously reported NUDT5-ADP-ribose complex structure illustrated extremely different binding modes of these substrates. Especially, unexpectedly, the positions of two phosphates of substrate ( and
and  phosphates) in the 8-oxo-dGDP complex are completely inverted compared to those in the ADP-ribose complex, which indicates that either the catalytic residues or the nucleophilic substitution sites of substrates are different between hydrolysis reactions of these substrates. To further investigate the catalytic mechanisms, we next determined the site of nucleophilic substitution of water for 8-oxo-dGDP and ADP-ribose by the
phosphates) in the 8-oxo-dGDP complex are completely inverted compared to those in the ADP-ribose complex, which indicates that either the catalytic residues or the nucleophilic substitution sites of substrates are different between hydrolysis reactions of these substrates. To further investigate the catalytic mechanisms, we next determined the site of nucleophilic substitution of water for 8-oxo-dGDP and ADP-ribose by the 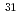 P NMR spectra of the reaction products in
P NMR spectra of the reaction products in  O-labeled water. The results clearly showed that the sites were different between these substrates, that is, 8-oxo-dGDP was attacked by a nucleophilic water at the P
O-labeled water. The results clearly showed that the sites were different between these substrates, that is, 8-oxo-dGDP was attacked by a nucleophilic water at the P , whereas ADP-ribose was at the P
, whereas ADP-ribose was at the P . Thus, we revealed the unique catalytic mechanism of NUDT5 as well as the multiple substrate recognition mechanism by structural and NMR analyses.
. Thus, we revealed the unique catalytic mechanism of NUDT5 as well as the multiple substrate recognition mechanism by structural and NMR analyses.