Structural and functional characterization of the 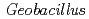 copper nitrite reductase; Involvement of the unique N-terminal region in the interprotein electron transfer with its redox partner
copper nitrite reductase; Involvement of the unique N-terminal region in the interprotein electron transfer with its redox partner
Fukuda, Yota*; Koteishi, Hiroyasu*; Yoneda, Ryohei*; Tamada, Taro; Takami, Hideto*; Inoue, Tsuyoshi*; Nojiri, Masaki
The crystal structures of copper-containing nitrite reductase (CuNiR) from the thermophilic Gram-positive bacterium 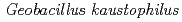 HTA426 and the amino (N)-terminal 68 residue-deleted mutant were determined at resolutions of 1.3
HTA426 and the amino (N)-terminal 68 residue-deleted mutant were determined at resolutions of 1.3 and 1.8
and 1.8 , respectively. Both structures show a striking resemblance with the overall structure of the well-known CuNiRs composed of two Greek key
, respectively. Both structures show a striking resemblance with the overall structure of the well-known CuNiRs composed of two Greek key  -barrel domains; however, a remarkable structural difference was found in the N-terminal region. The unique region has one
-barrel domains; however, a remarkable structural difference was found in the N-terminal region. The unique region has one  -strand and one
-strand and one  -helix extended to the northern surface of the type-1 copper site. The superposition of the
-helix extended to the northern surface of the type-1 copper site. The superposition of the 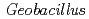 CuNiR model on the electron-transfer complex structure of CuNiR with the redox partner cytochrome
CuNiR model on the electron-transfer complex structure of CuNiR with the redox partner cytochrome 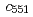 in other denitrifier system led us to infer that this region contributes to the transient binding with the partner protein during the interprotein electron transfer reaction in the
in other denitrifier system led us to infer that this region contributes to the transient binding with the partner protein during the interprotein electron transfer reaction in the 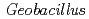 system. Furthermore, electron-transfer kinetics experiments using N-terminal residue-deleted mutant and the redox partner,
system. Furthermore, electron-transfer kinetics experiments using N-terminal residue-deleted mutant and the redox partner, 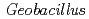 cytochrome
cytochrome 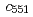 , were carried out. These structural and kinetics studies demonstrate that that region is directly involved in the specific partner recognition.
, were carried out. These structural and kinetics studies demonstrate that that region is directly involved in the specific partner recognition.