Sequential radiation chemical reactions in aqueous bromide solutions; Pulse radiolysis experiment and spur model simulation
Yamashita, Shinichi*; Iwamatsu, Kazuhiro; Maehashi, Yuki*; Taguchi, Mitsumasa; Hata, Kuniki  ; Muroya, Yusa*; Katsumura, Yosuke*
; Muroya, Yusa*; Katsumura, Yosuke*
Pulse radiolysis experiments were carried out to observe transient absorptions of reaction intermediates produced in N O
O and Ar-saturated aqueous solutions containing 0.9-900 mM NaBr. The most important species among the reaction intermediates are BrOH
and Ar-saturated aqueous solutions containing 0.9-900 mM NaBr. The most important species among the reaction intermediates are BrOH 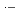 and Br
and Br
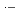 , which commonly have absorption peaks around 360 nm. The experimental results were compared with the results of simulation based on a spur diffusion model. Each of several complicated sequential radiation-induced chemical reactions was carefully considered, optimizing its rate constant within a range of reported values. All the experimental results were able to be universally reproduced by the simulation, assuming a reaction not yet reported, 2BrOH
, which commonly have absorption peaks around 360 nm. The experimental results were compared with the results of simulation based on a spur diffusion model. Each of several complicated sequential radiation-induced chemical reactions was carefully considered, optimizing its rate constant within a range of reported values. All the experimental results were able to be universally reproduced by the simulation, assuming a reaction not yet reported, 2BrOH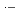
 Br
Br + 2OH
+ 2OH , with a rate constant of 3.8
, with a rate constant of 3.8  10
10 M
M s
s , which is significant only within 10 micro-s for rather high bromide concentrations (
, which is significant only within 10 micro-s for rather high bromide concentrations ( 10 mM). Primary
10 mM). Primary 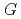 values, which are yields after sufficient diffusion from the spur to the perimeter region during 100 ns, of major water decomposition products, as well as of the reaction intermediates, were calculated for N
values, which are yields after sufficient diffusion from the spur to the perimeter region during 100 ns, of major water decomposition products, as well as of the reaction intermediates, were calculated for N O
O and Ar-saturated conditions as a function of NaBr concentration.
and Ar-saturated conditions as a function of NaBr concentration.