Sequential radiation chemical reactions in aqueous bromide solutions; Pulse radiolysis experiment and spur model simulation
ブロマイド水溶液中の放射線化学逐次反応;パルスラジオリシスとスパーモデルシミュレーション
山下 真一*; 岩松 和宏; 前橋 佑樹*; 田口 光正; 端 邦樹  ; 室屋 裕佐*; 勝村 庸介*
; 室屋 裕佐*; 勝村 庸介*
Yamashita, Shinichi*; Iwamatsu, Kazuhiro; Maehashi, Yuki*; Taguchi, Mitsumasa; Hata, Kuniki; Muroya, Yusa*; Katsumura, Yosuke*
ブロマイド(Br )は水酸化(OH)ラジカルと反応して分子吸光係数の大きな中間体を生じるため、放射線誘起水中OHラジカルの反応プローブとして使われてきた。放射線照射後ナノ秒領域のOHラジカルの挙動を解明するためにはBr
)は水酸化(OH)ラジカルと反応して分子吸光係数の大きな中間体を生じるため、放射線誘起水中OHラジカルの反応プローブとして使われてきた。放射線照射後ナノ秒領域のOHラジカルの挙動を解明するためにはBr の濃度を高くする必要があるものの、高濃度のBr
の濃度を高くする必要があるものの、高濃度のBr とOHラジカルの反応機構は不明であった。N
とOHラジカルの反応機構は不明であった。N OおよびArで飽和した0.9-900mMのNaBr水溶液へのパルス電子線照射によって生じたOHラジカルとBr
OおよびArで飽和した0.9-900mMのNaBr水溶液へのパルス電子線照射によって生じたOHラジカルとBr の反応中間体の時間挙動を光吸収により計測した。Br
の反応中間体の時間挙動を光吸収により計測した。Br はOHラジカルと反応してBrOH
はOHラジカルと反応してBrOH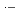 、さらにBr
、さらにBr
 を生じる。異なる実験条件で得られたBrOH
を生じる。異なる実験条件で得られたBrOH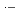 やBr
やBr
 のタイムプロファイルに対して、既報の反応速度式、速度定数を用いたスパーモデルシミュレーションを行った結果、10mM以上の高濃度条件では、2BrOH
のタイムプロファイルに対して、既報の反応速度式、速度定数を用いたスパーモデルシミュレーションを行った結果、10mM以上の高濃度条件では、2BrOH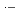
 Br
Br + 2OH
+ 2OH の反応(反応度度定数: k=3.8
の反応(反応度度定数: k=3.8 10
10 M
M s
s )を新たに考慮することで実験結果をよく再現できることを明らかにした。
)を新たに考慮することで実験結果をよく再現できることを明らかにした。
Pulse radiolysis experiments were carried out to observe transient absorptions of reaction intermediates produced in N O
O and Ar-saturated aqueous solutions containing 0.9-900 mM NaBr. The most important species among the reaction intermediates are BrOH
and Ar-saturated aqueous solutions containing 0.9-900 mM NaBr. The most important species among the reaction intermediates are BrOH 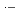 and Br
and Br
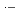 , which commonly have absorption peaks around 360 nm. The experimental results were compared with the results of simulation based on a spur diffusion model. Each of several complicated sequential radiation-induced chemical reactions was carefully considered, optimizing its rate constant within a range of reported values. All the experimental results were able to be universally reproduced by the simulation, assuming a reaction not yet reported, 2BrOH
, which commonly have absorption peaks around 360 nm. The experimental results were compared with the results of simulation based on a spur diffusion model. Each of several complicated sequential radiation-induced chemical reactions was carefully considered, optimizing its rate constant within a range of reported values. All the experimental results were able to be universally reproduced by the simulation, assuming a reaction not yet reported, 2BrOH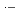
 Br
Br + 2OH
+ 2OH , with a rate constant of 3.8
, with a rate constant of 3.8  10
10 M
M s
s , which is significant only within 10 micro-s for rather high bromide concentrations (
, which is significant only within 10 micro-s for rather high bromide concentrations ( 10 mM). Primary
10 mM). Primary 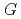 values, which are yields after sufficient diffusion from the spur to the perimeter region during 100 ns, of major water decomposition products, as well as of the reaction intermediates, were calculated for N
values, which are yields after sufficient diffusion from the spur to the perimeter region during 100 ns, of major water decomposition products, as well as of the reaction intermediates, were calculated for N O
O and Ar-saturated conditions as a function of NaBr concentration.
and Ar-saturated conditions as a function of NaBr concentration.