Evaluation of the resolvable capacity of Bragg reflections for a new diffractometer at J-PARC/MLF designed for protein crystals with large unit cells
Tomoyori, Katsuaki; Kurihara, Kazuo; Tamada, Taro; Kuroki, Ryota
We aim to build a high-resolution neutron time-of-flight diffractometer for biomacromolecules at the Materials and Life Science Experimental Facility (MLF) at the Japan Proton Accelerator Research Complex (J-PARC) that allows the collection of neutron diffraction data from crystals with unit cells of  250
250  ;. Considering both the flux and pulse width necessary to realize data collection covering a minimum d-spacing of 2.0
;. Considering both the flux and pulse width necessary to realize data collection covering a minimum d-spacing of 2.0  ; and with a unit cell constant of
; and with a unit cell constant of  250
250  ; we chose a decoupled moderator (DM) as the appropriate source for this high-resolution diffractometer. We considered a simple instrumentation model that includes a moderator, neutron guide, sample size, and neutron detector; we then investigated its spot separation performance and estimated the instrumental parameters for the design of a new diffractometer for protein crystals with large unit cells at J-PARC/MLF. It is preferable to extend the total flight path to resolve Bragg reflections for protein crystals with large unit cells as the scattering angle increases. Meanwhile, to ensure resolvable detection capacity at the middle scattering angle region (2
; we chose a decoupled moderator (DM) as the appropriate source for this high-resolution diffractometer. We considered a simple instrumentation model that includes a moderator, neutron guide, sample size, and neutron detector; we then investigated its spot separation performance and estimated the instrumental parameters for the design of a new diffractometer for protein crystals with large unit cells at J-PARC/MLF. It is preferable to extend the total flight path to resolve Bragg reflections for protein crystals with large unit cells as the scattering angle increases. Meanwhile, to ensure resolvable detection capacity at the middle scattering angle region (2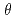
 90
90 ), it is necessary to restrict the angular divergence. In the case of
), it is necessary to restrict the angular divergence. In the case of 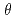

 0.2
0.2 , scattering angles from around 2
, scattering angles from around 2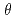
 90
90 to higher backscattering angles are more efficient for protein crystals with large unit cells (
to higher backscattering angles are more efficient for protein crystals with large unit cells ( 250
250  ) with a resolution of 2.0
) with a resolution of 2.0  .
.