Interaction of Fe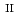 and Si under anoxic and reducing conditions; Structural characteristics of ferrous silicate co-precipitates
and Si under anoxic and reducing conditions; Structural characteristics of ferrous silicate co-precipitates
Francisco, P. C. M. 
 ; Mitsui, Seiichiro
; Mitsui, Seiichiro 
 ; Ishidera, Takamitsu
; Ishidera, Takamitsu  ; Tachi, Yukio
; Tachi, Yukio 
 ; Doi, Reisuke
; Doi, Reisuke 
 ; Shiwaku, Hideaki
; Shiwaku, Hideaki 


The interaction of Fe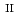 and S
and S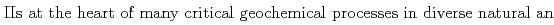 -silicate phases, which play important roles in regulating the solubility and bioavailability of both Fe
-silicate phases, which play important roles in regulating the solubility and bioavailability of both Fe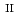 and Si, as well as serve as sinks for trace elements. Therefore, a detailed understanding of their structural characteristics may provide insights that may help in predicting their reactivity and stability under different conditions. In this work, co-precipitates with different Si/Fe
and Si, as well as serve as sinks for trace elements. Therefore, a detailed understanding of their structural characteristics may provide insights that may help in predicting their reactivity and stability under different conditions. In this work, co-precipitates with different Si/Fe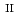 ratios (0.5, 1.0 and 2.0) were synthesized under anoxic and reducing conditions at different solution pH (7, 9 and 11). The co-precipitates were studied using X-ray diffraction (XRD), infrared (IR) spectroscopy and Fe
ratios (0.5, 1.0 and 2.0) were synthesized under anoxic and reducing conditions at different solution pH (7, 9 and 11). The co-precipitates were studied using X-ray diffraction (XRD), infrared (IR) spectroscopy and Fe  -edge X-ray absorption spectroscopy (XAS). The results show the immediate and rapid formation of phyllosilicate-like local structures from solution. These incipient structural units lack long-range order but may serve as the precursors of crystalline phases.
-edge X-ray absorption spectroscopy (XAS). The results show the immediate and rapid formation of phyllosilicate-like local structures from solution. These incipient structural units lack long-range order but may serve as the precursors of crystalline phases.