Phase-field model for crystallization in alkali disilicate glasses; Li O-2SiO
O-2SiO , Na
, Na O-2SiO
O-2SiO and K
and K O-2SiO
O-2SiO
Kawaguchi, Munemichi 

 ; Uno, Masayoshi*
; Uno, Masayoshi*
This study developed phase-field method (PFM) technique in oxide melt system by using a new mobility coefficient ( ). The crystal growth rates (
). The crystal growth rates (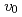 ) obtained by the PFM calculation with the constant
) obtained by the PFM calculation with the constant  were comparable to the thermodynamic driving force in normal growth model. The temperature dependence of the
were comparable to the thermodynamic driving force in normal growth model. The temperature dependence of the  was determined from the experimental crystal growth rates and the
was determined from the experimental crystal growth rates and the 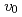 . Using the determined
. Using the determined  , the crystal growth rates (
, the crystal growth rates (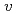 ) in alkali disilicate glasses, Li
) in alkali disilicate glasses, Li O-2SiO
O-2SiO , Na
, Na O-2SiO
O-2SiO and K
and K O-2SiO
O-2SiO were simulated. The temperature dependence of the
were simulated. The temperature dependence of the 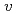 was qualitatively and quantitatively so similar that the PFM calculation results demonstrated the validity of the
was qualitatively and quantitatively so similar that the PFM calculation results demonstrated the validity of the  . Especially, the
. Especially, the 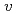 obtained by the PFM calculation appeared the rapid increase just below the thermodynamic melting point (
obtained by the PFM calculation appeared the rapid increase just below the thermodynamic melting point (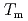 ) and the steep peak at around
) and the steep peak at around 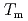 -100 K. Additionally, as the temperature decreased, the
-100 K. Additionally, as the temperature decreased, the 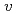 apparently approached zero ms
apparently approached zero ms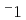 , which is limited by the
, which is limited by the  representing the interface jump process. Furthermore, we implemented the PFM calculation for the variation of the parameter
representing the interface jump process. Furthermore, we implemented the PFM calculation for the variation of the parameter  in the
in the  . As the
. As the  increased from zero to two, the peak of the
increased from zero to two, the peak of the 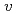 became steeper and the peak temperature of the
became steeper and the peak temperature of the 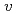 shifted to the high temperature side. The parameters
shifted to the high temperature side. The parameters 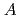 and
and  in the
in the  increased exponentially and decreased linearly as the atomic number of the alkali metal increased due to the ionic potential, respectively. This calculation revealed that the
increased exponentially and decreased linearly as the atomic number of the alkali metal increased due to the ionic potential, respectively. This calculation revealed that the 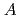 and
and  in the
in the  were close and reasonable for each other.
were close and reasonable for each other.