Observation of dihydrogen bonds in high-pressure phases of ammonia borane by X-ray and neutron diffraction measurements
Nakano, Satoshi*; Sano, Asami 

 ; Hattori, Takanori
; Hattori, Takanori 

 ; Machida, Shinichi*; Komatsu, Kazuki*; Fujihisa, Hiroshi*; Yamawaki, Hiroshi*; Goto, Yoshito*; Kikegawa, Takumi*
; Machida, Shinichi*; Komatsu, Kazuki*; Fujihisa, Hiroshi*; Yamawaki, Hiroshi*; Goto, Yoshito*; Kikegawa, Takumi*
X-ray and neutron diffraction analyses of ammonia borane were conducted at ambient and high pressures. The H-H distance in dihydrogen bonds was shorter than twice the van der Waals radius (2.4  ). The half of the dihydrogen bonds were broken on phase transition from AP to the first high pressure phase (HP1) at approximately 1.2 GPa as revealed by an increase in the H-H distances. On further pressure increase, all of the H-H distances became shorter than 2.4
). The half of the dihydrogen bonds were broken on phase transition from AP to the first high pressure phase (HP1) at approximately 1.2 GPa as revealed by an increase in the H-H distances. On further pressure increase, all of the H-H distances became shorter than 2.4  again, implying the pressure-induced reformation of the dihydrogen bonds. Furthermore, the HP1 transformed to the second one with the structure of
again, implying the pressure-induced reformation of the dihydrogen bonds. Furthermore, the HP1 transformed to the second one with the structure of 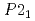 (Z = 2) at about 11 GPa. In this phase transition, the inclination of the molecule axis became larger and the number of types of dihydrogen bonds increased from 6 to 11. Just before the third transition at 18.9 GPa, the shortest dihydrogen bond decreased to 1.65
(Z = 2) at about 11 GPa. In this phase transition, the inclination of the molecule axis became larger and the number of types of dihydrogen bonds increased from 6 to 11. Just before the third transition at 18.9 GPa, the shortest dihydrogen bond decreased to 1.65  . The present study experimentally first confirmed the breakage and reformation of the dihydrogen bonds by the structural change under pressure.
. The present study experimentally first confirmed the breakage and reformation of the dihydrogen bonds by the structural change under pressure.