DXAFS study on laser-induced photoreduction mechanism of Rh ion complexes; Extraction of intermediates information by multivariate spectral analysis
ion complexes; Extraction of intermediates information by multivariate spectral analysis
Saeki, Morihisa*; Matsumura, Daiju 

 ; Nakanishi, Ryuzo*; Yomogida, Takumi
; Nakanishi, Ryuzo*; Yomogida, Takumi 

 ; Tsuji, Takuya
; Tsuji, Takuya 
 ; Saito, Hiroyuki*; Oba, Hironori*
; Saito, Hiroyuki*; Oba, Hironori* 

The precious metal (PM) ions complexed with negative ions and water molecules in solution has a charge transfer absorption band in the UV region. When alcohol is added to such a PM complex solution and irradiated with an ultraviolet laser, the PM
complex solution and irradiated with an ultraviolet laser, the PM complex is electronically excited and reacts with the alcohol to be reduced to the neutral atom PM
complex is electronically excited and reacts with the alcohol to be reduced to the neutral atom PM . The reduced PM
. The reduced PM spontaneously aggregates in the solution to form fine particles. This process is called Laser-Induced Particle Formation (LIPF), and is used for the formation of precious metal nanoparticles and the recovery of precious metals from factory effluents. We have investigated the LIPF reaction mechanism of Rh
spontaneously aggregates in the solution to form fine particles. This process is called Laser-Induced Particle Formation (LIPF), and is used for the formation of precious metal nanoparticles and the recovery of precious metals from factory effluents. We have investigated the LIPF reaction mechanism of Rh ion complexes by in situ energy-dispersive X-ray absorption fine structure spectroscopy(XAFS). As a result of analyzing the obtained XAFS spectra, we found that Rh species with three oxidation numbers are involved in the Rh
ion complexes by in situ energy-dispersive X-ray absorption fine structure spectroscopy(XAFS). As a result of analyzing the obtained XAFS spectra, we found that Rh species with three oxidation numbers are involved in the Rh reduction reaction, which proceeds from Rh
reduction reaction, which proceeds from Rh to Rh
to Rh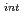 (intermediate) to Rh
(intermediate) to Rh .
.