Mechanisms responsible for adsorption of molybdate ions on alumina for the production of medical radioisotopes
Fujita, Yoshitaka 

 ; Niizeki, Tomotake*; Fukumitsu, Nobuyoshi*; Ariga, Katsuhiko*; Yamauchi, Yusuke*; Malgras, V.*; Kaneti, Y. V.*; Liu, C.-H.*; Hatano, Kentaro*; Suematsu, Hisayuki*; Suzuki, Tatsuya*; Tsuchiya, Kunihiko
; Niizeki, Tomotake*; Fukumitsu, Nobuyoshi*; Ariga, Katsuhiko*; Yamauchi, Yusuke*; Malgras, V.*; Kaneti, Y. V.*; Liu, C.-H.*; Hatano, Kentaro*; Suematsu, Hisayuki*; Suzuki, Tatsuya*; Tsuchiya, Kunihiko 
In this work, the mechanisms responsible for the adsorption of molybdate ions on alumina are investigated using in-depth surface analyses carried out on alumina specimens immersed in solutions containing different molybdate ions at different pH values. The obtained results reveal that when alumina is immersed in an acidic solution containing molybdate ions, the hydroxyl groups present on the surface are removed to generate positively charged sites, and molybdate ions (MoO
 or AlMo
or AlMo O
O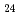 H
H
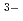 ) are adsorbed by electrostatic interaction. Alumina dissolves slightly in an acidic solution to form AlMo
) are adsorbed by electrostatic interaction. Alumina dissolves slightly in an acidic solution to form AlMo O
O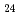 H
H
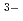 , which is more easily desorbed than MoO
, which is more easily desorbed than MoO
 . Furthermore, the enhancement in the Mo adsorption or desorption property may be achieved by enriching the surface of the alumina adsorbent with many -OH groups and optimizing Mo solution to adsorb molybdate ions on alumina as MoO
. Furthermore, the enhancement in the Mo adsorption or desorption property may be achieved by enriching the surface of the alumina adsorbent with many -OH groups and optimizing Mo solution to adsorb molybdate ions on alumina as MoO
 ions. These findings will assist researchers in engineering more efficient and stable alumina-based adsorbents for molybdenum adsorption used in medical radioisotope (
ions. These findings will assist researchers in engineering more efficient and stable alumina-based adsorbents for molybdenum adsorption used in medical radioisotope ( Mo/
Mo/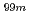 Tc) generators.
Tc) generators.