Conformation, hydration, and ligand exchange process of ruthenium nitrosyl complexes in aqueous solution; Free-energy calculations by a combination of molecular-orbital theories and different solvent models
QM/MM型方法論を用いた自由エネルギー計算による水溶液内に溶存したニトロシルルテニウム錯体の構造,水和,配位子交換反応に関する理論的研究
城戸 健太朗  ; 金子 政志
; 金子 政志 


Kido, Kentaro; Kaneko, Masashi
Distribution of solvent molecules near transition-metal complex is key information to comprehend the functionality, reactivity and so on. However, polarizable continuum solvent models still are the standard and conventional partner of molecular-orbital (MO) calculations in the solution system including transition-metal complex. In this study, we investigate the conformation, hydration structure and ligand substitution reaction between NO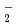 and H
and H O in aqueous solution for [Ru(NO)(OH)(NO
O in aqueous solution for [Ru(NO)(OH)(NO )
) ]
] (
(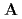 ), [Ru(NO)(OH)(NO
), [Ru(NO)(OH)(NO )
) (ONO)]
(ONO)] (
(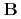 ) and [Ru(NO)(OH)(NO
) and [Ru(NO)(OH)(NO )
) (H
(H O)]
O)] (
( ) using a combination method of MO theories and a state-of-the-art molecular solvation technique (NI-MC-MOZ-SCF). In the complexes, the treatment is essentially required because except for nitrosyl ligand, a strong hydrogen bond is formed between the ligand and solvent water. These results are complementary to the data previously obtained by
) using a combination method of MO theories and a state-of-the-art molecular solvation technique (NI-MC-MOZ-SCF). In the complexes, the treatment is essentially required because except for nitrosyl ligand, a strong hydrogen bond is formed between the ligand and solvent water. These results are complementary to the data previously obtained by  N NMR experiment. A dominant species is found in the complex
N NMR experiment. A dominant species is found in the complex 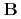 conformers and, as expected, different between the solvent models, which reveals that molecular solvation beyond continuum media treatment are required for a reliable description of solvation near transition-metal complex. In the stability constant evaluation of ligand substitution reaction, similar to the previous reports, an assumption that considers the direct association between the dissociated nitrite anion and complex
conformers and, as expected, different between the solvent models, which reveals that molecular solvation beyond continuum media treatment are required for a reliable description of solvation near transition-metal complex. In the stability constant evaluation of ligand substitution reaction, similar to the previous reports, an assumption that considers the direct association between the dissociated nitrite anion and complex  is useful to obtain a reliable stability constant.
is useful to obtain a reliable stability constant.