Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
岸 浩史*; 坂本 友和*; 朝澤 浩一郎*; 山口 進*; 加藤 豪士*; Zulevi, B.*; Serov, A.*; Artyushkova, K.*; Atanassov, P.*; 松村 大樹; et al.
Nanomaterials (Internet), 8(12), p.965_1 - 965_13, 2018/12
被引用回数:13 パーセンタイル:49.03(Chemistry, Multidisciplinary)Platinum group metal-free catalysts based on transition metal-nitrogen-carbon nanomaterials have been studied by a combination of in situ X-ray spectroscopy techniques, high-resolution transmission electron microscope, M ssbauer spectroscopy, electrochemical methods and density functional theory. Fe-N-C oxygen reduction reaction electrocatalysts were synthesized by varying several synthetic parameters to obtain nanomaterials with different composition and morphology. Associated with Fe-N
ssbauer spectroscopy, electrochemical methods and density functional theory. Fe-N-C oxygen reduction reaction electrocatalysts were synthesized by varying several synthetic parameters to obtain nanomaterials with different composition and morphology. Associated with Fe-N motive and the presence of Fe metallic particles in the electrocatalysts showed the clear differences in the variation of composition; processing and treatment conditions of sacrificial support method. From the results of material characterization; catalytic activity and theoretical studies; Fe metallic particles (coated with carbon) are main contributors into the HO
motive and the presence of Fe metallic particles in the electrocatalysts showed the clear differences in the variation of composition; processing and treatment conditions of sacrificial support method. From the results of material characterization; catalytic activity and theoretical studies; Fe metallic particles (coated with carbon) are main contributors into the HO
 generation.
generation.
 O
O /C hydrazine electrooxidation catalysts for anion exchange membrane fuel cells
/C hydrazine electrooxidation catalysts for anion exchange membrane fuel cells坂本 友和*; 増田 晃之*; 吉本 光児*; 岸 浩史*; 山口 進*; 松村 大樹; 田村 和久; 堀 彰宏*; 堀内 洋輔*; Serov, A.*; et al.
Journal of the Electrochemical Society, 164(4), p.F229 - F234, 2017/01
被引用回数:14 パーセンタイル:38.45(Electrochemistry)NiO/ Nb O
O /C (8:1), (4:1), (2:1), NiO/C, and Ni/C catalysts for hydrazine electrooxidation were synthesized by an evaporation drying method followed by thermal annealing. Prepared catalysts were characterized by X-ray diffraction (XRD), high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM), energy dispersive X-ray spectrometry (EDS), and X-ray absorption fine structure (XAFS). The highest catalytic activity in mentioned above reactionwas found for Ni/C, followed by: NiO/Nb
/C (8:1), (4:1), (2:1), NiO/C, and Ni/C catalysts for hydrazine electrooxidation were synthesized by an evaporation drying method followed by thermal annealing. Prepared catalysts were characterized by X-ray diffraction (XRD), high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM), energy dispersive X-ray spectrometry (EDS), and X-ray absorption fine structure (XAFS). The highest catalytic activity in mentioned above reactionwas found for Ni/C, followed by: NiO/Nb O
O /C (8:1), NiO/Nb
/C (8:1), NiO/Nb O
O /C (4:1). NiO/Nb
/C (4:1). NiO/Nb O
O /C (2:1) whiles NiO/C has almost no activity for hydrazine oxidation. It was explained by oxygen defect of NiO in NiO/ Nb
/C (2:1) whiles NiO/C has almost no activity for hydrazine oxidation. It was explained by oxygen defect of NiO in NiO/ Nb O
O /C from XAFS analysis. The selectivity hydrazine electrooxidation as measured by ammonia production resulted in observation that metallic Ni surface facilitates N-N bond breaking of hydrazine, which was confirmed by density functional theory (DFT) calculations.
/C from XAFS analysis. The selectivity hydrazine electrooxidation as measured by ammonia production resulted in observation that metallic Ni surface facilitates N-N bond breaking of hydrazine, which was confirmed by density functional theory (DFT) calculations.
坂本 友和*; 岸 浩史*; 山口 進*; 松村 大樹; 田村 和久; 堀 彰宏*; 堀内 洋輔*; Serov, A.*; Artyushkova, K.*; Atanassov, P.*; et al.
Journal of the Electrochemical Society, 163(10), p.H951 - H957, 2016/08
被引用回数:33 パーセンタイル:75.37(Electrochemistry)The catalytic process takes place on nickel oxide surface of a Ni oxide nano-particle decorated carbon support (NiO/C). In-situ X-ray absorption fine structure (XAFS) spectroscopy was used to investigate the reaction mechanism for hydrazine electrooxidation on NiO surface. The spectra of X-ray absorption near-edge structure (XANES) of Ni K-edge indicated that adsorption of OH on Ni site during the hydrazine electrooxidation reaction. Density functional theory (DFT) calculations were used to elucidate and suggest the mechanism of the electrooxidation and specifically propose the localization of electron density from OH
on Ni site during the hydrazine electrooxidation reaction. Density functional theory (DFT) calculations were used to elucidate and suggest the mechanism of the electrooxidation and specifically propose the localization of electron density from OH to 3d orbital of Ni in NiO. It is found that the accessibility of Ni atomic sites in NiO structure is critical for hydrazine electrooxidation. Based on this study, we propose a possible reaction mechanism for selective hydrazine electrooxidation to water and nitrogen taking place on NiO surface as it is applicable to direct hydrazine alkaline membrane fuel cells.
to 3d orbital of Ni in NiO. It is found that the accessibility of Ni atomic sites in NiO structure is critical for hydrazine electrooxidation. Based on this study, we propose a possible reaction mechanism for selective hydrazine electrooxidation to water and nitrogen taking place on NiO surface as it is applicable to direct hydrazine alkaline membrane fuel cells.
坂本 友和*; 松村 大樹; 朝澤 浩一郎*; Martinez, U.*; Serov, A.*; Artyushkova, K.*; Atanassov, P.*; 田村 和久; 西畑 保雄; 田中 裕久*
Electrochimica Acta, 163, p.116 - 122, 2015/05
被引用回数:60 パーセンタイル:82.36(Electrochemistry)Carbon supported Ni, Ni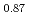 Zn
Zn , and Co hydrazine electrooxidation catalysts were synthesized by an impregnation/freeze-drying procedure followed by thermal annealing for use as anode catalyst of direct hydrazine hydrate fuel cells (DHFCs). The cell performance of DHFCs changed significantly when different catalysts were used as anode. Ammonia generation from anode outlet at open circuit voltage (OCV) condition was higher for Co/C than for Ni-based catalysts. To better understand the cause of different performance and selectivity of each anode catalyst, extensive ex-situ and operando characterization was carried out. Operando XAFS measurement of Ni-K and Co-
, and Co hydrazine electrooxidation catalysts were synthesized by an impregnation/freeze-drying procedure followed by thermal annealing for use as anode catalyst of direct hydrazine hydrate fuel cells (DHFCs). The cell performance of DHFCs changed significantly when different catalysts were used as anode. Ammonia generation from anode outlet at open circuit voltage (OCV) condition was higher for Co/C than for Ni-based catalysts. To better understand the cause of different performance and selectivity of each anode catalyst, extensive ex-situ and operando characterization was carried out. Operando XAFS measurement of Ni-K and Co- edge shows the potential dependence of atomic structure of Ni/C, Ni
edge shows the potential dependence of atomic structure of Ni/C, Ni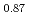 Zn
Zn /C, and Co/C during hydrazine electrooxidation reaction.
/C, and Co/C during hydrazine electrooxidation reaction.