Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Aoyama, Takahito; Choudhary, S.*; Pandaleon, A.*; Burns, J. T.*; Kokaly, M.*; Restis, J.*; Ross, J.*; Kelly, R. G.*
Corrosion, 81(6), p.609 - 621, 2025/06
Aoyama, Takahito; Ueno, Fumiyoshi; Sato, Tomonori; Kato, Chiaki; Sano, Naruto; Yamashita, Naoki; Otani, Kyohei; Igarashi, Takahiro
Annals of Nuclear Energy, 214, p.111229_1 - 111229_6, 2025/05
Times Cited Count:0 Percentile:0.00(Nuclear Science & Technology)Igarashi, Takahiro; Sugawara, Yu*; Otani, Kyohei; Aoyama, Takahito
Tetsu To Hagane, 110(15), p.1244 - 1250, 2024/11
Times Cited Count:0 Percentile:0.00(Metallurgy & Metallurgical Engineering)Using two types of image processing techniques without machine learning, edge extraction processing and keypoint extraction processing, progressively corroded regions under rust layer from images of corroded steel surfaces was extracted. We found that there is a relatively good correlation between the keypoint strength obtained from the keypoint extraction processing for HSL transformed and histogram flattened corroded surface photographs and the corrosion depth after removing rust removal.
Yamashita, Naoki; Aoyama, Takahito; Kato, Chiaki; Sano, Naruto; Tagami, Susumu
JAEA-Technology 2023-028, 22 Pages, 2024/03
At the Fukushima Daiichi Nuclear Power Station (1F), which is currently undergoing decommissioning, there is growing interest in the effects of radiation-emitting radionuclides such as  Sr and
Sr and  Cs on the structural integrity. In particular, the corrosion behavior of carbon steel, which is used in many parts of 1F, is known to change depending on metal cations in solution, but the effects of
Cs on the structural integrity. In particular, the corrosion behavior of carbon steel, which is used in many parts of 1F, is known to change depending on metal cations in solution, but the effects of  Sr and
Sr and  Cs on corrosion are not yet understood. In addition, it is important to investigate the distribution of
Cs on corrosion are not yet understood. In addition, it is important to investigate the distribution of  Sr and
Sr and  Cs in the rust layer in order to understand the corrosion behavior, but the method has not yet been established. In this study, a glove box was prepared to conduct corrosion tests of carbon steel in NaCl containing
Cs in the rust layer in order to understand the corrosion behavior, but the method has not yet been established. In this study, a glove box was prepared to conduct corrosion tests of carbon steel in NaCl containing  Sr and
Sr and  Cs in the glove box. In addition, in order to clarify the influence of
Cs in the glove box. In addition, in order to clarify the influence of  Sr and
Sr and  Cs, which exist as metal cations in the solution, on the corrosion behavior of carbon steel, we attempted to establish a detection method for radioactive materials in the rust layer using an imaging plate.
Cs, which exist as metal cations in the solution, on the corrosion behavior of carbon steel, we attempted to establish a detection method for radioactive materials in the rust layer using an imaging plate.
 Sr and
Sr and  Cs in HCl solutions on the corrosion potential of Type 316L stainless steel
Cs in HCl solutions on the corrosion potential of Type 316L stainless steelAoyama, Takahito; Sato, Tomonori; Ueno, Fumiyoshi; Kato, Chiaki; Sano, Naruto; Yamashita, Naoki; Igarashi, Takahiro
Zairyo To Kankyo, 72(11), p.284 - 288, 2023/11
no abstracts in English
 to the inside of the crevice by chelation and its effect on crevice corrosion of Type 316L stainless steel
to the inside of the crevice by chelation and its effect on crevice corrosion of Type 316L stainless steelAoyama, Takahito; Kato, Chiaki
Corrosion Science, 210(2), p.110850_1 - 110850_10, 2023/01
Times Cited Count:7 Percentile:43.24(Materials Science, Multidisciplinary)The chelated complex of Cu : [Cu(EDTA)]
: [Cu(EDTA)] was used to introduce Cu
was used to introduce Cu from outside to the inside of crevice. The introduced Cu
from outside to the inside of crevice. The introduced Cu was expected to act as an inhibitor for the crevice corrosion on stainless steels. Crevice corrosion tests, confirmed the introduction of Cu
was expected to act as an inhibitor for the crevice corrosion on stainless steels. Crevice corrosion tests, confirmed the introduction of Cu to the inside of the crevice via electromigration of [Cu(EDTA)]
to the inside of the crevice via electromigration of [Cu(EDTA)] was confirmed. Migrated [Cu(EDTA)]
was confirmed. Migrated [Cu(EDTA)] reacted with H
reacted with H and inhibited decrease in pH inside the crevice, where Cu
and inhibited decrease in pH inside the crevice, where Cu was separated from [Cu(EDTA)]
was separated from [Cu(EDTA)] and suppressed active dissolution of the stainless steel.
and suppressed active dissolution of the stainless steel.
 Sr dissolved solution on corrosion potential of type 316L stainless steel
Sr dissolved solution on corrosion potential of type 316L stainless steelAoyama, Takahito; Kato, Chiaki; Sato, Tomonori; Sano, Naruto; Yamashita, Naoki; Ueno, Fumiyoshi
Zairyo To Kankyo, 71(4), p.110 - 115, 2022/04
no abstracts in English
 in NaCl
in NaClAoyama, Takahito; Ogawa, Hiroaki; Kato, Chiaki; Ueno, Fumiyoshi
Metals, 11(3), p.511_1 - 511_13, 2021/03
Times Cited Count:3 Percentile:15.96(Materials Science, Multidisciplinary)The effect of Cu in bulk solution on pitting corrosion resistance of extra high purity type 316 stainless steel was investigated. Pitting occurred in 0.1 M NaCl-1 mM CuCl
in bulk solution on pitting corrosion resistance of extra high purity type 316 stainless steel was investigated. Pitting occurred in 0.1 M NaCl-1 mM CuCl whereas pitting was not initiated in 0.1 M NaCl. Although deposition of Cu
whereas pitting was not initiated in 0.1 M NaCl. Although deposition of Cu on the surface occurred regardless of potential region in 0.1 M NaCl-1 mM CuCl
on the surface occurred regardless of potential region in 0.1 M NaCl-1 mM CuCl , Cu
, Cu in bulk solution had no influence on the passive film formation. The decrease in pitting corrosion resistance in 0.1 M NaCl-1 mM CuCl
in bulk solution had no influence on the passive film formation. The decrease in pitting corrosion resistance in 0.1 M NaCl-1 mM CuCl resulted from the deposited Cu or Cu compound and continuous supply of Cu
resulted from the deposited Cu or Cu compound and continuous supply of Cu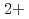 on the surface.
on the surface.

 generation; The Role of NO
generation; The Role of NO
 in the crevice corrosion repassivation of type 316L stainless steel
in the crevice corrosion repassivation of type 316L stainless steelAoyama, Takahito; Sugawara, Yu*; Muto, Izumi*; Hara, Nobuyoshi*
Journal of the Electrochemical Society, 166(10), p.C250 - C260, 2019/01
Times Cited Count:6 Percentile:12.85(Electrochemistry)The role of NO
 in the repassivation of crevice corrosion of Type 316L stainless steel was investigated. In crevice corrosion tests, the solution was changed from 1 M NaCl to NaCl-NaNO
in the repassivation of crevice corrosion of Type 316L stainless steel was investigated. In crevice corrosion tests, the solution was changed from 1 M NaCl to NaCl-NaNO . NO
. NO
 led to complete repassivation. Repassivation of the crevice corrosion was found to take place in two steps. In the first step, the estimated current density inside the crevice gradually decreased from ca. 5 mA cm
led to complete repassivation. Repassivation of the crevice corrosion was found to take place in two steps. In the first step, the estimated current density inside the crevice gradually decreased from ca. 5 mA cm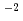 to ca. 5
to ca. 5  A cm
A cm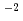 . After that, the current density suddenly decreased to less than 0.1
. After that, the current density suddenly decreased to less than 0.1  A cm
A cm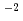 . From the potentiodynamic polarization in acidic solutions simulated inside the crevice (pH 0.2) and in situ observations of the crevice corrosion morphology, the first step was thought to be generated by the suppression of active dissolution by NO
. From the potentiodynamic polarization in acidic solutions simulated inside the crevice (pH 0.2) and in situ observations of the crevice corrosion morphology, the first step was thought to be generated by the suppression of active dissolution by NO
 . It would appear that the generation of NH
. It would appear that the generation of NH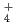 results in a pH increase and the further suppression of active dissolution, and then repassivation occurs.
results in a pH increase and the further suppression of active dissolution, and then repassivation occurs.
 on pitting corrosion of the extra high purity type 316 stainless steel
on pitting corrosion of the extra high purity type 316 stainless steelAoyama, Takahito; Ogawa, Hiroaki; Ueno, Fumiyoshi
no journal, ,
Aoyama, Takahito; Sato, Tomonori; Ueno, Fumiyoshi; Kato, Chiaki; Sano, Naruto; Yamashita, Naoki; Igarashi, Takahiro
no journal, ,
no abstracts in English
Igarashi, Takahiro; Otani, Kyohei; Aoyama, Takahito; Kato, Chiaki
no journal, ,
It is one of the important issues to understand the corrosion condition inside the material nondestructively in the integrity assessment of large structures such as bridges and plants. In this presentation, we attempted to determine the corrosion state from the surface image before rust removal by using the contour extraction method based on the discontinuity of luminance in the neighboring pixels of the image and the feature point extraction method using the gradient of the corner and luminance of the neighboring pixels. As a result, it was found that the Adaptive Gaussian and Canny methods could be used for contour extraction, and the Agast Feature Detector method could be used for feature point extraction to predict the vicinity of corrosion points.
Igarashi, Takahiro; Otani, Kyohei; Aoyama, Takahito
no journal, ,
Evaluation of the integrity of large structures such as bridges and plants is one of the most important issues. In order to understand the corrosion mechanisms of steels using these large structures and to apply them to the integrity evaluation, many studies have been conducted, including exposure tests and repeated wet and dry tests in the laboratory. In addition, another research approach has been conducted to predict the corrosion state from surface images, which is considered to be one of the most useful methods to predict the amount of corrosion of actual materials that cannot be destructively inspected. In this study, we show that the FAST feature point extraction method can be used to predict the location of corrosion, although it is difficult to predict the absolute value of corrosion depth, by applying the FAST feature point extraction method to surface images of carbon steel specimens that have been subjected to repeated wet and dry tests before rust removal.
Aoyama, Takahito; Takahatake, Yoko; Ito, Azusa; Ueno, Fumiyoshi; Okada, Takashi; Nagaishi, Ryuji; Koma, Yoshikazu
no journal, ,
Aoyama, Takahito; Sato, Tomonori; Ueno, Fumiyoshi; Kato, Chiaki; Sano, Naruto; Yamashita, Naoki; Igarashi, Takahiro
no journal, ,
no abstracts in English
Igarashi, Takahiro; Otani, Kyohei; Aoyama, Takahito
no journal, ,
Corrosion areas were predicted using the contour extraction methods (Global, Adaptive mean (AM), Adaptive Gaussian (AG), Canny) and feature point extraction methods (KAZE, AKAZE, BRISK, ORB, AFD, FAST, MSER, SBD) on surface images of carbon steel specimens that had been subjected to repeated dry and wet tests before rust removal, and it was confirmed that both methods could approximately extract relatively heavily corroded areas. In particular, it was confirmed that the feature point extraction method can extract intense corrosion areas.
Choudhary, S.*; Aoyama, Takahito; Pandaleon, A.*; Burns, J. T.*; Kokaly, M.*; Restis, J.*; Ross, J.*; Kelly, R. G.*
no journal, ,
 on crevice corrosion of type 316L stainless steel
on crevice corrosion of type 316L stainless steelAoyama, Takahito; Kato, Chiaki
no journal, ,
Alloyed Cu is known to inhibit the growth of pitting corrosion on stainless steels after pitting initiation. The Cu dissolved from the stainless steel matrix acts as an inhibitor in acidic chloride environments which is formed in pits. The Cu
dissolved from the stainless steel matrix acts as an inhibitor in acidic chloride environments which is formed in pits. The Cu suppresses active dissolution rate inside the pits
suppresses active dissolution rate inside the pits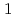 . This suppression effect is supposed to be also effective for active dissolution which promotes crevice corrosion. Therefore, if it is possible to introduce Cu
. This suppression effect is supposed to be also effective for active dissolution which promotes crevice corrosion. Therefore, if it is possible to introduce Cu to the inside of a crevice from the outside, introduced Cu
to the inside of a crevice from the outside, introduced Cu is supposed to inhibit crevice corrosion on stainless steel. However, diffusion of ions between inside and outside of the crevice is restricted by its geometry, and Cu
is supposed to inhibit crevice corrosion on stainless steel. However, diffusion of ions between inside and outside of the crevice is restricted by its geometry, and Cu does not migrate to inside of a crevice according to the electroneutrality principle. [Cu (EDTA (ethylenediaminetetraacetic acid))]
does not migrate to inside of a crevice according to the electroneutrality principle. [Cu (EDTA (ethylenediaminetetraacetic acid))] is a chelated Cu
is a chelated Cu which has negative charge, and is expected to migrate to inside of a crevice from the outside by electrochemical migration. In addition, it is reported that Cu
which has negative charge, and is expected to migrate to inside of a crevice from the outside by electrochemical migration. In addition, it is reported that Cu in [Cu(EDTA)]
in [Cu(EDTA)] could be easily substituted by Fe
could be easily substituted by Fe in low pH
in low pH . Therefore, Cu
. Therefore, Cu is considered to be introduced to the inside of the crevice as [Cu(EDTA)]
is considered to be introduced to the inside of the crevice as [Cu(EDTA)] , and to affect crevice corrosion of stainless steel. In this study, in situ observation of an inside of the crevice
, and to affect crevice corrosion of stainless steel. In this study, in situ observation of an inside of the crevice was performed to analyze the effect of [Cu(EDTA)]
was performed to analyze the effect of [Cu(EDTA)] on crevice corrosion.
on crevice corrosion.
 on crevice corrosion of Type 316L stainless steel
on crevice corrosion of Type 316L stainless steelAoyama, Takahito; Kato, Chiaki
no journal, ,
Crevice corrosion tests were performed on SUS 316L stainless steel using a flow cell that allows in-situ observation of the inside of the crevice. The inside of the crevice was filled with 0.1 M NaCl, and 0.1 M NaCl and 0.1 M NaCl-10 mM [Cu(EDTA)]Na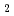 were used for the outside solution. The results showed that the time required for crevice corrosion to occur in 0.1 M NaCl-10 mM [Cu(EDTA)]Na
were used for the outside solution. The results showed that the time required for crevice corrosion to occur in 0.1 M NaCl-10 mM [Cu(EDTA)]Na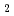 was longer than that in 0.1 M NaCl. The propagation behavior of crevice corrosion was also different. These results suggest that Cu(EDTA)
was longer than that in 0.1 M NaCl. The propagation behavior of crevice corrosion was also different. These results suggest that Cu(EDTA) suppressed the initiation and propagation of crevice corrosion.
suppressed the initiation and propagation of crevice corrosion.