Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Takahashi, Atsushi*; Chiba, Mirei*; Tanahara, Akira*; Aida, Jun*; Shimizu, Yoshinaka*; Suzuki, Toshihiko*; Murakami, Shinobu*; Koarai, Kazuma; Ono, Takumi*; Oka, Toshitaka; et al.
Scientific Reports (Internet), 11(1), p.10355_1 - 10355_11, 2021/05
Times Cited Count:9 Percentile:39.53(Multidisciplinary Sciences)Kitazato, Kohei*; Milliken, R. E.*; Iwata, Takahiro*; Abe, Masanao*; Otake, Makiko*; Matsuura, Shuji*; Takagi, Yasuhiko*; Nakamura, Tomoki*; Hiroi, Takahiro*; Matsuoka, Moe*; et al.
Nature Astronomy (Internet), 5(3), p.246 - 250, 2021/03
Times Cited Count:60 Percentile:96.18(Astronomy & Astrophysics)Here we report observations of Ryugu's subsurface material by the Near-Infrared Spectrometer (NIRS3) on the Hayabusa2 spacecraft. Reflectance spectra of excavated material exhibit a hydroxyl (OH) absorption feature that is slightly stronger and peak-shifted compared with that observed for the surface, indicating that space weathering and/or radiative heating have caused subtle spectral changes in the uppermost surface. However, the strength and shape of the OH feature still suggests that the subsurface material experienced heating above 300  C, similar to the surface. In contrast, thermophysical modeling indicates that radiative heating does not increase the temperature above 200
C, similar to the surface. In contrast, thermophysical modeling indicates that radiative heating does not increase the temperature above 200  C at the estimated excavation depth of 1 m, even if the semimajor axis is reduced to 0.344 au. This supports the hypothesis that primary thermal alteration occurred due to radiogenic and/or impact heating on Ryugu's parent body.
C at the estimated excavation depth of 1 m, even if the semimajor axis is reduced to 0.344 au. This supports the hypothesis that primary thermal alteration occurred due to radiogenic and/or impact heating on Ryugu's parent body.
Kikuchi, Tatsuya; Nakajima, Kenji; Kawamura, Seiko; Inamura, Yasuhiro; Yamamuro, Osamu*; Kofu, Maiko*; Kawakita, Yukinobu; Suzuya, Kentaro; Nakamura, Mitsutaka; Arai, Masatoshi
Physical Review E, 87(6), p.062314_1 - 062314_8, 2013/06
Times Cited Count:21 Percentile:73.28(Physics, Fluids & Plasmas)A quasi-elastic neutron scattering (QENS) experiment is a particular technique that endeavors to define a relationship between time and space for the diffusion dynamics of atoms and molecules. However, in most cases, analyses of QENS data are model dependent. We have developed a new method for processing QENS data without a specific model, wherein all modes can be described as combinations of the relaxations based on the exponential law. By this method, we can obtain a new distribution function, 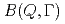 , which we call the mode distribution function (MDF), to represent the number of relaxation modes and distributions of the relaxation times in the modes. The deduction of MDF is based on the maximum entropy method. We report the first application to experimental data of liquid water. In addition to the two known modes, the existence of a new relaxation mode of water molecules with an intermediate time scale has been discovered.
, which we call the mode distribution function (MDF), to represent the number of relaxation modes and distributions of the relaxation times in the modes. The deduction of MDF is based on the maximum entropy method. We report the first application to experimental data of liquid water. In addition to the two known modes, the existence of a new relaxation mode of water molecules with an intermediate time scale has been discovered.
Nakamura, Kazuo*; Jiang, Y.*; Liu, X.*; Mitarai, Osamu*; Kurihara, Kenichi; Kawamata, Yoichi; Sueoka, Michiharu; Hasegawa, Makoto*; Tokunaga, Kazutoshi*; Zushi, Hideki*; et al.
Fusion Engineering and Design, 86(6-8), p.1080 - 1084, 2011/10
Times Cited Count:5 Percentile:36.74(Nuclear Science & Technology)Sato, Fuminori; Terunuma, Hitoshi*; Arai, Osamu*; Myochin, Munetaka
Nihon Genshiryoku Gakkai Wabun Rombunshi, 8(1), p.83 - 94, 2009/03
Oxide conversion using water vapor and boron oxide (B O
O ) was studied to treat salt waste from dry reprocessing. Parameter tests to CsCl and NaCl-2CsCl salt were performed and fundamental data such as conversion rate, etc. were acquired. To understand the process behavior, a reaction analysis based on thermodynamic equilibrium calculation considering salt (NaCl, CsCl), oxide (Na
) was studied to treat salt waste from dry reprocessing. Parameter tests to CsCl and NaCl-2CsCl salt were performed and fundamental data such as conversion rate, etc. were acquired. To understand the process behavior, a reaction analysis based on thermodynamic equilibrium calculation considering salt (NaCl, CsCl), oxide (Na O, Cs
O, Cs O, B
O, B O
O ) and gas (H
) and gas (H O, Ar, HCl, NaCl, CsCl) phase was performed. The validity of analysis was confirmed by comparison with the experiment. Using this result, process condition of the oxide conversion (ex. temperature, added amount of H
O, Ar, HCl, NaCl, CsCl) phase was performed. The validity of analysis was confirmed by comparison with the experiment. Using this result, process condition of the oxide conversion (ex. temperature, added amount of H O and B
O and B O
O , etc.) was discussed.
, etc.) was discussed.
Kurihara, Kenichi; Kawamata, Yoichi; Sueoka, Michiharu; Wang, F.*; Nakamura, Kazuo*; Mitarai, Osamu*; Sato, Konosuke*; Zushi, Hideki*; Hanada, Kazuaki*; Sakamoto, Mizuki*; et al.
Kyushu Daigaku Oyo Rikigaku Kenkyujo RIAM Fuoramu 2008 Koen Yoshi, p.66 - 69, 2008/06
no abstracts in English
Takase, Yuichi*; Ide, Shunsuke; Kamada, Yutaka; Kubo, Hirotaka; Mitarai, Osamu*; Nuga, Hideo*; Sakamoto, Yoshiteru; Suzuki, Takahiro; Takenaga, Hidenobu; JT-60 Team
Proceedings of 21st IAEA Fusion Energy Conference (FEC 2006) (CD-ROM), 8 Pages, 2007/03
A self-sustained state driven by the bootstrap current was achieved in JT-60U. Based on a high confinement reversed magnetic shear plasma, a plasma in which the bootstrap current frafciton of almost 100 percent (plasma current  0.55 MA) was successfully maintained for 2.5 s. The dynamic response of a completely self-driven system with negligible external current drive was studied. Repetition of beta collapses and recoveries were often observed. Furthermore, discharges in which the bootstrap current fraction can be expected to be larger than 100 percent were obtained.
0.55 MA) was successfully maintained for 2.5 s. The dynamic response of a completely self-driven system with negligible external current drive was studied. Repetition of beta collapses and recoveries were often observed. Furthermore, discharges in which the bootstrap current fraction can be expected to be larger than 100 percent were obtained.
Wang, F.*; Nakamura, Kazuo*; Mitarai, Osamu*; Kurihara, Kenichi; Kawamata, Yoichi; Sueoka, Michiharu; Sato, Konosuke*; Zushi, Hideki*; Hanada, Kazuaki*; Sakamoto, Mizuki*; et al.
Kyushu Daigaku Oyo Rikigaku Kenkyujo RIAM Fuoramu 2006 Koen Yoshi, p.138 - 141, 2006/06
no abstracts in English
Shirai, Osamu*; Yamana, Hajimu*; Arai, Yasuo
Journal of Alloys and Compounds, 408-412, p.1267 - 1273, 2006/02
Times Cited Count:44 Percentile:84.93(Chemistry, Physical)no abstracts in English
Ushigome, Masahiro*; Ide, Shunsuke; Ito, Satoshi*; Jotaki, Eriko*; Mitarai, Osamu*; Shiraiwa, Shunichi*; Suzuki, Takahiro; Takase, Yuichi*; Tanaka, Shigetoshi*; Fujita, Takaaki; et al.
Nuclear Fusion, 46(2), p.207 - 213, 2006/02
Times Cited Count:12 Percentile:38.16(Physics, Fluids & Plasmas)This papaer studies on tokamak plasma start-up completely without central solenoid (CS). On the JT-60 tokamak it is demonstrated that a completely CS-less Ip start-up to 100 kA was achieved even without any null-point by Electron cyclotron range of frequencies (ECRF) and outer PF coil current swing only. Necessary conditions (the EC power, the toroidal field etc.) were clarified. Moreover, it was succeded to maintain Ip = 260kA for 1 sec without CS by NB only. In addition Ip ramp-up by EC and NB only (without LHCD) from 215 to 310kA was achieved. In a high confinement reversed shear discharge, a result suggesting bootstrap over drive was obtained.
Sato, Fuminori; Myochin, Munetaka; Terunuma, Hitoshi*; Arai, Osamu*
Proceedings of International Conference on Nuclear Energy System for Future Generation and Global Sustainability (GLOBAL 2005) (CD-ROM), 4 Pages, 2005/10
Oxide conversion of molten salt wastes in pyrochemical reprocessing was studied as a pre-treatment of vitrification. A new method using boron oxide and water vapor was suggested from consideration of a conventional method using boric acid (H3BO3) by chemical equilibrium calculation. Applying the new method for NaCl-CsCl salt in a small-scale experiment, it was confirmed that the most of the salt was converted to oxide and the amount of the oxide wastes after the treatment could be reduced by 20% compared with the conventional method.
Shirai, Osamu; Kato, Tetsuya*; Iwai, Takashi; Arai, Yasuo; Yamashita, Toshiyuki
Journal of Physics and Chemistry of Solids, 66(2-4), p.456 - 460, 2005/02
Times Cited Count:8 Percentile:36.09(Chemistry, Multidisciplinary)Electrochemical behaviors of PuN and (U, Pu)N in the LiCl-KCl eutectic melt containing UCl and PuCl
and PuCl at 773 K were investigated by cyclic voltammetry. The electrochemical dissolution of PuN and (U, Pu)N began nearly at -1.0 V vs. the Ag/AgCl reference electrode. The rest potentials of PuN and (U, Pu)N were observed at about 0.15 V more negative potential than that of UN since the equilibrium potential of UN is about 0.15 V more positive than that of PuN. In the cyclic voltammogram measured by using (U, Pu)N as the working electrode, a steep rise of the positive current was observed at more positive potential than -0.4 V in analogy with the cyclic voltammogram measured by using UN as the working electrode. In addition, there were two anodic current waves in the voltammogram with (U, Pu)N, though the wave form was not clear. This indicates that UN and PuN would be dissolved independently irrespective of formation of the solid solution, (U, Pu)N.
at 773 K were investigated by cyclic voltammetry. The electrochemical dissolution of PuN and (U, Pu)N began nearly at -1.0 V vs. the Ag/AgCl reference electrode. The rest potentials of PuN and (U, Pu)N were observed at about 0.15 V more negative potential than that of UN since the equilibrium potential of UN is about 0.15 V more positive than that of PuN. In the cyclic voltammogram measured by using (U, Pu)N as the working electrode, a steep rise of the positive current was observed at more positive potential than -0.4 V in analogy with the cyclic voltammogram measured by using UN as the working electrode. In addition, there were two anodic current waves in the voltammogram with (U, Pu)N, though the wave form was not clear. This indicates that UN and PuN would be dissolved independently irrespective of formation of the solid solution, (U, Pu)N.
 /Np couple at liquid Cd and Bi electrodes in LiCl-KCl eutectic melts
/Np couple at liquid Cd and Bi electrodes in LiCl-KCl eutectic meltsShirai, Osamu; Uozumi, Koichi*; Iwai, Takashi; Arai, Yasuo
Journal of Applied Electrochemistry, 34(3), p.323 - 330, 2004/03
Times Cited Count:29 Percentile:52.76(Electrochemistry)The electrode reactions of the Np /Np couple at liquid Cd and Bi electrodes were investigated by cyclic voltammetry at 723, 773 and 823 K in LiCl-KCl eutectic melt. It was found that the diffusion of Np
/Np couple at liquid Cd and Bi electrodes were investigated by cyclic voltammetry at 723, 773 and 823 K in LiCl-KCl eutectic melt. It was found that the diffusion of Np in the salt phase was a rate-determining step in the cathodic reaction when the concentration of NpCl
in the salt phase was a rate-determining step in the cathodic reaction when the concentration of NpCl was less than about 1 wt.% and the liquid Cd or Bi phase was not saturated with Np. The redox potentials of the Np
was less than about 1 wt.% and the liquid Cd or Bi phase was not saturated with Np. The redox potentials of the Np /Np couple at liquid Cd electrode at 723, 773 and 823 K were observed more positively than those at Mo electrode by 0.158, 0.140 and 0.126 V, respectively. The potential shift would result from a lowering of activity of Np in Cd phase according to the alloy formation of NpCd
/Np couple at liquid Cd electrode at 723, 773 and 823 K were observed more positively than those at Mo electrode by 0.158, 0.140 and 0.126 V, respectively. The potential shift would result from a lowering of activity of Np in Cd phase according to the alloy formation of NpCd at 723 K and NpCd
at 723 K and NpCd at 773 and 823 K. The redox potentials of the Np
at 773 and 823 K. The redox potentials of the Np /Np couple at liquid Bi electrode at 723, 773 and 823 K were more positive than those at Mo electrode by 0.427, 0.419 and 0.410 V, respectively, which would be attributable to a lowering of activity of Np in Bi phase according to the formation of NpBi
/Np couple at liquid Bi electrode at 723, 773 and 823 K were more positive than those at Mo electrode by 0.427, 0.419 and 0.410 V, respectively, which would be attributable to a lowering of activity of Np in Bi phase according to the formation of NpBi .
.
Terunuma, Hitoshi*; Arai, Osamu*
JNC TJ8400 2004-037, 73 Pages, 2004/02
In order to convert a salt waste from dry reprocessing to oxide for vitrification, a basic examination about the conversion reaction of chloride was performed. The salt waste simulator (chloride salt) was reacted with boron oxide and water vapor at 1023K-1123K, and converted to oxide with occurring of hydrogen chloride as by-product. the hydrogen chloride generated in this reaction was stabilized by hydrogen chloride stabilizer
Uozumi, Koichi*; Iizuka, Masatoshi*; Kato, Tetsuya*; Inoue, Tadashi*; Shirai, Osamu*; Iwai, Takashi; Arai, Yasuo
Journal of Nuclear Materials, 325(1), p.34 - 43, 2004/02
Times Cited Count:113 Percentile:98.53(Materials Science, Multidisciplinary)Experiments were conducted on simultaneous recovering of uranium and plutonium electrochemically into laboratory scale liquid cadomium cathodes(LCCs) at different U/Pu ratios in the salt phase. The influence of the salt composition on the recovered amount of uranium and plutonium, the morphology of uranium and plutonium in the LCCs, and the behavior of americium were examined. It was shouwn that there is a threshold in the U/Pu ratio in the salt phase for the successful simultaneous recovery of uranium and plutonium up to 10wt% in high current efficiencies.
Shiraiwa, Shunichi*; Ide, Shunsuke; Ito, Satoshi*; Mitarai, Osamu*; Naito, Osamu; Ozeki, Takahisa; Sakamoto, Yoshiteru; Suzuki, Takahiro; Takase, Yuichi*; Tanaka, Shigetoshi*; et al.
Physical Review Letters, 92(3), p.035001_1 - 035001_4, 2004/01
Times Cited Count:52 Percentile:84.02(Physics, Multidisciplinary)no abstracts in English
Sato, Fuminori; Myochin, Munetaka; Terunuma, Hitoshi*; Arai, Osamu*
Nihon Genshiryoku Gakkai Wabun Rombunshi, 3(3), p.307 - 311, 2004/00
Out-of-pile experiments on the release of fission products (FPs) under transient heating conditions were carried out for mixed oxide fuels. The fuels used in the experiments, the plutonium content of which was 30wt%, were irradiated up to 65 GWd/t in the experimental fast reactor JOYO. The experiments consisted of two runs, FP-1 and FP-2. In FP-1, the fuel sample was first heated to 2,000 C and then up to 3,000
C and then up to 3,000 C. The holding time was 30 min at each temperature. In FP-2, the terminal temperatures were 1,500
C. The holding time was 30 min at each temperature. In FP-2, the terminal temperatures were 1,500 C and 2,500
C and 2,500 C, and the holding time was 30 min in the same manner. The release of Cs, a volatile FP, was detected as soon as the fuel sample was heated up. The release rate, after peaking in several minutes, decreased gradually via a diffusion process in the fuel matrix. The "gross" diffusion coefficient agreed well with the data reported in other experiments using LWR fuels. The release fractions were identical in both experiments, namely Cs 100%, Sb 100%, Ru 10%, Ce 0% and Eu 0%.
C, and the holding time was 30 min in the same manner. The release of Cs, a volatile FP, was detected as soon as the fuel sample was heated up. The release rate, after peaking in several minutes, decreased gradually via a diffusion process in the fuel matrix. The "gross" diffusion coefficient agreed well with the data reported in other experiments using LWR fuels. The release fractions were identical in both experiments, namely Cs 100%, Sb 100%, Ru 10%, Ce 0% and Eu 0%.
Kato, Tetsuya*; Uozumi, Koichi*; Inoue, Tadashi*; Shirai, Osamu*; Iwai, Takashi; Arai, Yasuo
Proceedings of GLOBAL2003 Atoms for Prosperity; Updating Eisenhower's Global Vision for Nuclear Energy (CD-ROM), p.1591 - 1595, 2003/11
Electrolysis experiments were carried to recover plutonium and uranium into liquid cadmium cathodes from molten salt at high cathode current densities. In the electrolysis at 101mA/cm , 10.4wt.% of heavy metals in the cathode was recovered at almost 100% of current efficiency. In the electrolysis at 156mA/cm
, 10.4wt.% of heavy metals in the cathode was recovered at almost 100% of current efficiency. In the electrolysis at 156mA/cm , the cathode potential ascended after approximately 8wt.% of heavy metals was recovered and some deposit was observed outside of the crucible.
, the cathode potential ascended after approximately 8wt.% of heavy metals was recovered and some deposit was observed outside of the crucible.
Shirai, Osamu*; Yamana, Hajimu*; Iwai, Takashi; Arai, Yasuo
Proceedings of Nuclear Fuel Cycle Technologies Closing the Fuel Cycle (CD-ROM), 7 Pages, 2003/00
no abstracts in English
Shirai, Osamu; Uozumi, Koichi*; Iwai, Takashi; Arai, Yasuo
Journal of Nuclear Science and Technology, 39(Suppl.3), p.745 - 748, 2002/11
no abstracts in English