Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 in a genuine dissolver solution from the results of extraction simulation by PARC-L code
in a genuine dissolver solution from the results of extraction simulation by PARC-L codeAsakura, Toshihide; Hotoku, Shinobu; Morita, Yasuji
Journal of Nuclear Science and Technology, 52(12), p.1552 - 1561, 2015/12
Times Cited Count:1 Percentile:8.75(Nuclear Science & Technology)With a genuine spent fuel soltuion (a dissolver solution), a laboratory-scale reprocessing experiment of an extraction-separation process was performed using mixer-settlers as extactors. In the experiment, n-butyraldehyde was utilized as a reducing reagent of Np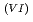 O
O
 to Np
to Np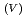 O
O
 for the purpose to distinguish Np
for the purpose to distinguish Np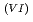 O
O
 from Np
from Np . From the Np concentration in the aqueous phase, Np would be extracted from the dissolver solution together with U and Pu. The scrutiny of Np behavior was performed utilizing 66 cases of calculation results by a Japan Atomic Energy Agency open extraction simulation code, the Programm for Advanced Extraction with Radiation Effect Calculation-Lightened version. From the scrutiny, the authors found that the calculation result with 60% of Np
. From the Np concentration in the aqueous phase, Np would be extracted from the dissolver solution together with U and Pu. The scrutiny of Np behavior was performed utilizing 66 cases of calculation results by a Japan Atomic Energy Agency open extraction simulation code, the Programm for Advanced Extraction with Radiation Effect Calculation-Lightened version. From the scrutiny, the authors found that the calculation result with 60% of Np in the dissolver solution represented the best experimental extraction-separtion behavior of Np. Therefore, it was supposed that the dissolver solution contained sufficient proportion of Np
in the dissolver solution represented the best experimental extraction-separtion behavior of Np. Therefore, it was supposed that the dissolver solution contained sufficient proportion of Np to affect the extraction-separation behavior of Np.
to affect the extraction-separation behavior of Np.
Hoshi, Harutaka; Kikuchi, Takahiro; Asakura, Toshihide; Morita, Yasuji; Kimura, Takaumi
JAEA-Research 2010-016, 70 Pages, 2010/07
We have studied selective separation of Cs and Sr, which are included in high level liquid waste (HLLW) generated from reprocessing of spent nuclear fuel and are major heat generators, by using extractant impregnated adsorbents. Cs adsorbent using calix arane derivatives showed excellent selectivity for Cs. It also showed significant stability against  -irradiation. Sr adsorbent using crown ether derivatives also showed high selectivity for Sr from nitric acid solution, except for Ba and Tc. Dynamic capacity decreased ca. 30% after
-irradiation. Sr adsorbent using crown ether derivatives also showed high selectivity for Sr from nitric acid solution, except for Ba and Tc. Dynamic capacity decreased ca. 30% after  -irradiation. Hot test using genuine HLLW stored in NUCEF was performed for separation of Cs and Sr through columns, respectively. Each Cs and Sr was separated from other typical fission product elements as well as the results obtained in preliminary experiments. Finally, Cs and Sr were separated according to a supposed separation scheme. Although some complexing agents were added in simulate HLLW, no negative effect was found.
-irradiation. Hot test using genuine HLLW stored in NUCEF was performed for separation of Cs and Sr through columns, respectively. Each Cs and Sr was separated from other typical fission product elements as well as the results obtained in preliminary experiments. Finally, Cs and Sr were separated according to a supposed separation scheme. Although some complexing agents were added in simulate HLLW, no negative effect was found.
Kikuchi, Takahiro; Hoshi, Harutaka; Asakura, Toshihide; Morita, Yasuji; Kimura, Takaumi; Dodbiba, G.*; Fujita, Toyohisa*
JAEA-Research 2010-010, 45 Pages, 2010/07
We have investigate that separation of Mo from simulated HLLW using various metal oxides adsorbent. Fe-Pb oxides and manganese oxide showed very high solubility in nitric acid solution. The distribution coefficient of Mo was decreased with increasing nitric acid concentration among tested adsorbents. Adsorption ability of Mo on alumina and cobalt oxide was low in 3M nitric acid. Hematite type iron oxide (Fe adsorbent) and amorphous zirconium oxide had high Mo adsorption ability, in 3M nitric acid. TRU, U and major fission products were not adsorbed on the adsorbent. So, separation of Mo can be achieved by using Fe adsorbent. A part of Mo was adsorbed irreversibly on Fe adsorbent, but reversibly-adsorbed Mo was recovered by oxalic acid, and the adsorbent was able to use repeatedly. Behavior of break-through of Mo is estimated from adsorption isotherm and overall mass transfer coefficient. We found that amount of throughput of Mo increased with decreasing grain size of the adsorbent.
Morita, Yasuji; Sasaki, Yuji; Asakura, Toshihide; Kitatsuji, Yoshihiro; Sugo, Yumi; Kimura, Takaumi
IOP Conference Series; Materials Science and Engineering, 9, p.012057_1 - 012057_11, 2010/05
Times Cited Count:8 Percentile:91.58(Chemistry, Inorganic & Nuclear)The authors have developed a new type of extractant for Am and Cm,  -tetraoctyl-diglycolamide (TODGA), which gives very high distribution ratio at relatively high nitric acid concentration. To apply such a new extractant to the separation process for Am and Cm from HLLW, many criteria should be investigated and satisfied; e.g., separability against fission products (FP), chemical and radiolytic stability, extraction capacity, compatibility with hydrocarbon diluents, and so on. From a viewpoint of extraction capacity, TODGA is modified to
-tetraoctyl-diglycolamide (TODGA), which gives very high distribution ratio at relatively high nitric acid concentration. To apply such a new extractant to the separation process for Am and Cm from HLLW, many criteria should be investigated and satisfied; e.g., separability against fission products (FP), chemical and radiolytic stability, extraction capacity, compatibility with hydrocarbon diluents, and so on. From a viewpoint of extraction capacity, TODGA is modified to  -tetradodecyl-diglycolamide (TDdDGA). Since TDdDGA extract Zr and Pd, effective masking agents for them were examined and selected. With those achievements, a counter-current extraction test with 0.1 M TDdDGA in n-dodecane was carried out using a small-scale mixer-settler and simulated solution of HLLW. As results of the test, a very high recovery of Am, more than 99.96%, was obtained and good separation from FP was observed.
-tetradodecyl-diglycolamide (TDdDGA). Since TDdDGA extract Zr and Pd, effective masking agents for them were examined and selected. With those achievements, a counter-current extraction test with 0.1 M TDdDGA in n-dodecane was carried out using a small-scale mixer-settler and simulated solution of HLLW. As results of the test, a very high recovery of Am, more than 99.96%, was obtained and good separation from FP was observed.
 -tetradodecyl-diglycolamide
-tetradodecyl-diglycolamideSasaki, Yuji; Asakura, Toshihide; Kitatsuji, Yoshihiro; Morita, Yasuji; Kimura, Takaumi
Proceedings of 10th OECD/NEA Information Exchange Meeting on Actinide and Fission Product Partitioning and Transmutation (CD-ROM), 6 Pages, 2010/00
Diglycolamide, DGA, compounds have the strong extractability with trivalent, tetravalent and hexavalent actinides (An) from nitric acid to n-dodecane. Since DGA connecting with the long alkyl chain, TDdDGA ( -tetradodecyl-diglycolamide), has the high extraction capacity and suppresses to form the third phase with Nd, we used TDdDGA to demonstrate the counter-current extraction, prior to the hot test. In this experiment we use four elements, i.e., Sr, Pd, Zr and Nd, as the typical fission products and a representative ion of An(III) and lanthanides, to confirm the extraction and the separation properties, these four elements are extractable by TDdDGA. After determination of the optimum condition on the extraction and separation of Nd from other elements by the calculation using D values, we perform the counter-current extraction using the mixer-settler equipment.
-tetradodecyl-diglycolamide), has the high extraction capacity and suppresses to form the third phase with Nd, we used TDdDGA to demonstrate the counter-current extraction, prior to the hot test. In this experiment we use four elements, i.e., Sr, Pd, Zr and Nd, as the typical fission products and a representative ion of An(III) and lanthanides, to confirm the extraction and the separation properties, these four elements are extractable by TDdDGA. After determination of the optimum condition on the extraction and separation of Nd from other elements by the calculation using D values, we perform the counter-current extraction using the mixer-settler equipment.
Tsubata, Yasuhiro; Asakura, Toshihide; Morita, Yasuji
Nihon Genshiryoku Gakkai Wabun Rombunshi, 8(3), p.211 - 220, 2009/09
A computer code PARC was developed in JAEA, in order to predict behavior of U, Pu, Np and several fission products in a reprocessing plant for spent nuclear fuel. This code has several useful calculation functions, i.e., PARC can deal with both mixer-settlers and pulsed columns simultaneously, and new equations for chemical reaction, reaction constant and distribution ratio can be easily inputted to PARC without recompilation of the program. Therefore it is expected to be widely used for basic solvent extraction study, separation process design, plant management and safety analysis for future reprocessing. In the present, outline of a basic extractor model and several chemical reaction models used in PARC were introduced, and results of calculations for U and Pu behavior in U/Pu separation process in PUREX were shown, which elucidated the effect of the increase in Pu concentration in the feed solution and the importance of the selection of Pu oxidation/reduction reaction models.
Koma, Yoshikazu; Sano, Yuichi; Morita, Yasuji; Asakura, Toshihide
Proceedings of International Conference on Advanced Nuclear Fuel Cycle; Sustainable Options & Industrial Perspectives (Global 2009) (CD-ROM), p.1056 - 1060, 2009/09
The chromatography technology has been investigated for actinides (An) (III) separation from a highly acidic solution as a part of reprocessing system for spent fast breeder reactor fuel. The adsorbent for the chromatography is based on the porous silica support impregnated with an extractant that is used for solvent extraction. Several extractants, CMPO, TODGA, HDEHP and BTP, were examined for the two steps flowsheet; An(III)/lanthanides (Ln) (III) recovery and An(III)/Ln(III) separation. The CMPO and TODGA adsorbents are the potential candidate from the chromatogram of Am, Cm and some fission product elements for An(III)-Ln(III) recovery whereas the HDEHP and BTP adsorbents are for An(III)/Ln(III) separation. The regenerated adsorbents after removing and re-impregnating the extractant show the identical adsorption property to the original.
Ban, Yasutoshi; Asakura, Toshihide; Morita, Yasuji
Journal of Radioanalytical and Nuclear Chemistry, 279(2), p.423 - 429, 2009/02
Times Cited Count:18 Percentile:71.49(Chemistry, Analytical)Reduction kinetics of Pu(IV) by N,N-dimethylhydrazine (NNDMH) were studied by spectrophotometry, and the reduction rate equation in 3 M (mol/dm ) nitric acid was obtained as follows: -d[Pu(IV)]
) nitric acid was obtained as follows: -d[Pu(IV)]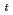 /d
/d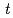 =
=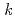 [NNDMH][Pu(IV)] where
[NNDMH][Pu(IV)] where 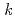 =8.3
=8.3 10
10 M
M s
s at room temperature (298
at room temperature (298 1 K). Reduction properties of NNDMH for U(VI), Np(VI), and Pu(IV) was studied in the mixture solution of tri-n-butylphosphate diluted to 30 vol.% by n-dodecane (30% TBP) and 3 M nitric acid. It was confirmed that NNDMH selectively reduce Np(VI) to Np(V) without affecting the valences of U(VI) and Pu(IV) in a few minutes. Simple numerical simulation indicated that 99.9% of Np was separated from U and Pu applying NNDMH for a mixer-settler.
1 K). Reduction properties of NNDMH for U(VI), Np(VI), and Pu(IV) was studied in the mixture solution of tri-n-butylphosphate diluted to 30 vol.% by n-dodecane (30% TBP) and 3 M nitric acid. It was confirmed that NNDMH selectively reduce Np(VI) to Np(V) without affecting the valences of U(VI) and Pu(IV) in a few minutes. Simple numerical simulation indicated that 99.9% of Np was separated from U and Pu applying NNDMH for a mixer-settler.
Ban, Yasutoshi; Hagiya, Hiromichi; Sato, Makoto; Asakura, Toshihide; Morita, Yasuji
Proceedings of 3rd International ATALANTE Conference (ATALANTE 2008) (CD-ROM), 4 Pages, 2008/05
Since N,N-dialkylamide (monoamide) compounds extract tetravalent and hexavalent actinides, they have been proposed as alternative extractants to TBP. In the present work, numerical calculations for estimating separation performance of N,N-di(2-ethyl)hexyl-butanamide (D2EHBA) for U(VI) and Pu(IV) were carried out. A flow sheet was obtained which separate more than 99.9% of Pu(IV) from U(VI) by adjusting nitric acid concentration. Extraction properties of D2EHBA for macro concentrations of U (0.63-1.22 mol/dm (M)) and Pu (6.3 mM) were studied in a batch manner. D2EHBA diluted to 1.5 M by n-dodecane extracted up to 0.8 M of U(VI) without forming precipitation and third phase. Distribution ratios of Pu(IV) were relatively high compared with the ones obtained at tracer concentrations of Pu(IV).
(M)) and Pu (6.3 mM) were studied in a batch manner. D2EHBA diluted to 1.5 M by n-dodecane extracted up to 0.8 M of U(VI) without forming precipitation and third phase. Distribution ratios of Pu(IV) were relatively high compared with the ones obtained at tracer concentrations of Pu(IV).
Koma, Yoshikazu; Watanabe, So; Sano, Yuichi; Asakura, Toshihide; Morita, Yasuji
Proceedings of 3rd International ATALANTE Conference (ATALANTE 2008) (CD-ROM), 8 Pages, 2008/05
Tsubata, Yasuhiro; Asakura, Toshihide; Morita, Yasuji
JAEA-Data/Code 2008-010, 145 Pages, 2008/04
A computer code PARC was developed for simulating liquid-liquid extraction process in the PUREX reprocessing plant. PARC is able to predict transient behavior and profiles at equilibrium of uranium, plutonium, neptunium and fission products in several units of pulsed columns and mixer-settlers, which are connected each other in the PUREX plant. In this report, mathematical models of mass transfer and chemical reactions employed in PARC are described and an example of PUREX simulation is given.
Hoshi, Harutaka*; Wei, Y.-Z.*; Kumagai, Mikio*; Asakura, Toshihide; Morita, Yasuji
Journal of Alloys and Compounds, 444-445, p.663 - 667, 2007/10
Times Cited Count:4 Percentile:32.34(Chemistry, Physical)no abstracts in English
Sasaki, Yuji; Kitatsuji, Yoshihiro; Sugo, Yumi; Asakura, Toshihide; Kimura, Takaumi
Proceedings of International Conference on Advanced Nuclear Fuel Cycles and Systems (Global 2007) (CD-ROM), p.1117 - 1120, 2007/09
An innovative chemical separation process for the treatment of high level radioactive liquid waste (HLW) has been developed in our laboratory. There are two main stages in this process. The diglycolamide (DGA) compounds employed for TRU recovery have several advantages, (1) carbon, hydrogen, oxygen and nitrogen atoms in their structures and gasification by combustion, (2) easy synthesis, (3) neutral and tridentate ligands, and (4) strong extraction ability with An from HNO ). DGA compounds with the different structures can be obtained by attachment of the different alkyl groups to amidic N atoms, i.e., tetraoctyl-diglycolamide (TODGA), tetradodecyl-diglycolamide (TDdDGA), tetrametyl-diglycolamide (TMDGA) and tetraetyl-diglycolamide (TEDGA). The utility of DGA compounds, which shows the different characters, is revealed and the flow-sheet based on the salt-free concept is exhibited in this paper.
). DGA compounds with the different structures can be obtained by attachment of the different alkyl groups to amidic N atoms, i.e., tetraoctyl-diglycolamide (TODGA), tetradodecyl-diglycolamide (TDdDGA), tetrametyl-diglycolamide (TMDGA) and tetraetyl-diglycolamide (TEDGA). The utility of DGA compounds, which shows the different characters, is revealed and the flow-sheet based on the salt-free concept is exhibited in this paper.
Ban, Yasutoshi; Asakura, Toshihide; Morita, Yasuji
JAEA-Research 2006-076, 22 Pages, 2006/11
Extraction behavior of U and Pu by N,N-di-(2-ethyl)hexylbutanamide (D2EHBA) in a mixer-settler type extractor was analyzed by a simple calculation code. Analysis was carried out with the consideration of acid and element concentration effects on the distribution ratios of U and Pu. A flow-sheet that gives the separation of U and Pu by adjusting nitric acid concentration without the use of Pu reductant was obtained. According to the analysis based on this flow sheet, U/Pu ratio in the Pu product stream, the ratio of Pu in raffinate, and the ratio of Pu in the Pu product stream were 1.06, less than 0.1%, and more than 99.9%, respectively.
Imaizumi, Hirobumi; Ban, Yasutoshi; Asakura, Toshihide; Morita, Yasuji
JAEA-Technology 2006-043, 45 Pages, 2006/09
In JAEA, the solvent washing properties of n-butylamine compounds, which can be decomposed by incineration or electrolysis, have been investigated using simulated and real degraded tributylphosphate (TBP) solvent. Ion chromatography has been utilized as an analytical method to determine the concentration of dibutylphosphoric acid (DBP) in organic and aqueous phases. Recently, we met difficulty to maintain the reliability of analytical results. A gas chromatograph-mass spectroscope (GC-MS) was considered as new analytical method to solve these problems. As a result, it was confirmed that improved reliability of analysis can be obtained by utilizing a sample pre-treatment method to introduce tetra methyl silyl substituent to the target molecule, DBP. In a chromatogram, monobutylphosphoric acid also gave good peaks. I can be expected to analyze DBP and MBP simultaneously with only one sample in the TBP solvent.
Hoshi, Harutaka*; Wei, Y.*; Kumagai, Mikio*; Asakura, Toshihide; Morita, Yasuji
Recent Advances in Actinide Science, p.596 - 598, 2006/06
Recently, extraction selectivity for trivalent minor actinides (MA = Am and Cm) over lanthanides (Ln) has been found in some extractants containing soft donor, such as S or N, ligands. Kolarik et al. reported that a new N-donor ligand 2,6-bis(5,6-dialkyl-1,2,4-triazine-3-yl)-pyridine (R-BTP) shows high selectivity for MA (III) over Ln(III) [1]. However, protonation of R-BTP results in its acidic hydrolysis in acidic medium. Stability in acidic solution was improved by substitution of long normal chain or branched chain [2]. In this work, separation of MA(III) and Ln(III) from nitric acid solution was studied by using novel R-BTP impregnated resin. Branched R-BTP resin had high affinity for Am from up to 4 M HNO solution and its distribution coefficient was over 10
solution and its distribution coefficient was over 10 .
.
 -dimethylformamide
-dimethylformamideKim, S.-Y.; Asakura, Toshihide; Morita, Yasuji; Ikeda, Yasuhisa*
Journal of Alloys and Compounds, 408-412, p.1291 - 1295, 2006/02
Times Cited Count:10 Percentile:53.71(Chemistry, Physical)The electrochemical reactions of UO (
( -diketonato)
-diketonato) DMF, UO
DMF, UO (trop)
(trop) DMF and UO
DMF and UO (sap)(DMF)
(sap)(DMF) , (DMF = N,N-dimethylformamide,
, (DMF = N,N-dimethylformamide,  -diketonate = thenoyltrifluoroacetonate (ttfa), benzoyltrifluoroacetonate (btfa), and dibenzoylmethanate(dbm), trop = tropolonate, and sap = 2-salicylidenaminophenolate) complexes in DMF solution containing tetrabutyl ammonium perchlorate as a supporting electrolyte have been studied with cyclic voltammetry. These uranyl(VI) complexes were found to be quasi-reversibly reduced to U(V) species. The formal redox potentials (E
-diketonate = thenoyltrifluoroacetonate (ttfa), benzoyltrifluoroacetonate (btfa), and dibenzoylmethanate(dbm), trop = tropolonate, and sap = 2-salicylidenaminophenolate) complexes in DMF solution containing tetrabutyl ammonium perchlorate as a supporting electrolyte have been studied with cyclic voltammetry. These uranyl(VI) complexes were found to be quasi-reversibly reduced to U(V) species. The formal redox potentials (E ,
, 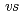 . ferrocene/ferrocenium) for U(VI)/U(V) couples were determined to be -1.18 V for UO
. ferrocene/ferrocenium) for U(VI)/U(V) couples were determined to be -1.18 V for UO (ttfa)
(ttfa) DMF, -1.18 V for UO
DMF, -1.18 V for UO (btfa)
(btfa) DMF, -1.46 V for UO
DMF, -1.46 V for UO (dbm)
(dbm) DMF, -1.46 V for UO
DMF, -1.46 V for UO (trop)
(trop) DMF, and -1.59 V for UO
DMF, and -1.59 V for UO (sap)(DMF)
(sap)(DMF) .
.
Hoshi, Harutaka*; Wei, Y.*; Kumagai, Mikio*; Asakura, Toshihide; Morita, Yasuji
Journal of Alloys and Compounds, 408-412, p.1274 - 1277, 2006/02
Times Cited Count:40 Percentile:83.91(Chemistry, Physical)For the development of advanced aqueous reprocessing system, it is one of the most important subjects to separate minor trivalent actinides (MA = Am and Cm). Recently, extraction selectivity for MA(III) over Ln(III) has been found in some extractants containing soft donor, such as S or N, ligands. Kolarik et al. reported that a new N-donor ligand 2,6-bis(5,6-dialkyl-1,2,4-triazine-3-yl)-pyridine (R-BTP) shows high selectivity for MA (III) over Ln(III). The novel silica-based extraction resins were prepared by impregnating some R-BTP molecules into a macroreticular styrene-divinylbenzene copolymer which is immobilized in porous silica particles with a mean diameter of 50  m. Separation of simulated high level liquid waste solution containing Ln(III) and trace amount of Am(III) was studied. Am(III) was mutually separated from Ln(III) through a packed column with R-BTP impregnating resin, very high decontamination factor (
m. Separation of simulated high level liquid waste solution containing Ln(III) and trace amount of Am(III) was studied. Am(III) was mutually separated from Ln(III) through a packed column with R-BTP impregnating resin, very high decontamination factor ( 10
10 ) for Am, and all the elements were recovered quantitatively.
) for Am, and all the elements were recovered quantitatively.
Asakura, Toshihide; Hotoku, Shinobu; Ban, Yasutoshi; Matsumura, Masakazu; Morita, Yasuji
Journal of Nuclear and Radiochemical Sciences, 6(3), p.271 - 274, 2005/12
Tc extraction and separation experiments were performed basing on PUREX technique with using spent UO fuel with burn-up of 44 GWd/t. The experimental results were examined with performing calculations by a simulation code ESSCAR (Extraction System Simulation Code for Advanced Reprocessing). It was demonstrated that Tc can be almost quantitatively extracted from a dissolver solution and that Tc can also be almost quantitatively recovered by scrubbing. Further, it was clearly presented from the calculation results of ESSCAR that the extraction mechanism of Tc is dominated by the synergistic effect of Zr and U.
fuel with burn-up of 44 GWd/t. The experimental results were examined with performing calculations by a simulation code ESSCAR (Extraction System Simulation Code for Advanced Reprocessing). It was demonstrated that Tc can be almost quantitatively extracted from a dissolver solution and that Tc can also be almost quantitatively recovered by scrubbing. Further, it was clearly presented from the calculation results of ESSCAR that the extraction mechanism of Tc is dominated by the synergistic effect of Zr and U.
Asakura, Toshihide; Kim, S.-Y.; Morita, Yasuji; Ozawa, Masaki*
Journal of Nuclear and Radiochemical Sciences, 6(3), p.267 - 269, 2005/12
An electrolytic extraction (EE) method, i.e. electro-reductive deposition, of Tc from nitric acid aqueous solution was studied for future reprocessing. After 30 min of constant potential electrolysis by carbon electrode at -0.3 V vs. SSE (Standard Silver Electrode), Tc concentration in 3 M nitric acid decreased to 93 % of the initial value, which corresponds to 7 % of deposition. With co-existence of Pd, the value reached to 15 % of deposition equivalent by electrolysis at  0.0 V vs. SSE for 60 min. An acceleration effect of Pd on Tc deposition (promoter effect) was suggested. The concentration, however, increased to the initial value after further electrolysis and competing re-dissolution of deposited Tc was also suggested. In cyclic voltammetry measurements, it was found that the deposit from Tc-Pd-Ru-Rh solution dissolved easier than that from Pd-Ru-Rh did. In electrolyzed Tc solution, an absorption peak at 482 nm was found. It can be attributed to the complex with nitrite anion, and the complex formation is proposed as one possible mechanism of Tc re-dissolution.
0.0 V vs. SSE for 60 min. An acceleration effect of Pd on Tc deposition (promoter effect) was suggested. The concentration, however, increased to the initial value after further electrolysis and competing re-dissolution of deposited Tc was also suggested. In cyclic voltammetry measurements, it was found that the deposit from Tc-Pd-Ru-Rh solution dissolved easier than that from Pd-Ru-Rh did. In electrolyzed Tc solution, an absorption peak at 482 nm was found. It can be attributed to the complex with nitrite anion, and the complex formation is proposed as one possible mechanism of Tc re-dissolution.