Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 At] astatine
At] astatineBalkin, E. R.*; Hamlin, D. K.*; Gagnon, K.*; Chyan, M.-K.*; Pal, S.*; 渡辺 茂樹; Wilbur, D. S.*
Applied Sciences (Internet), 3(3), p.636 - 655, 2013/09
被引用回数:39 パーセンタイル:86.55(Chemistry, Multidisciplinary)A "wet chemistry" approach for isolation of  At from an irradiated bismuth target is described. The approach involves five steps: (1) dissolution of bismuth target in conc. HNO
At from an irradiated bismuth target is described. The approach involves five steps: (1) dissolution of bismuth target in conc. HNO ; (2) removal of the HNO
; (2) removal of the HNO by distillation; (3) dissolution of residue in 8 M HCl; (4) extraction of
by distillation; (3) dissolution of residue in 8 M HCl; (4) extraction of  At from 8 M HCl into DIPE; and (5) extraction of
At from 8 M HCl into DIPE; and (5) extraction of  At from DIPE into NaOH. Results from 55 "optimized" At isolation runs gave recovery yields of approximately 78% after decay and attenuation corrections. An attenuation-corrected average of 26
At from DIPE into NaOH. Results from 55 "optimized" At isolation runs gave recovery yields of approximately 78% after decay and attenuation corrections. An attenuation-corrected average of 26 3 mCi in the target provided isolated (actual) yields of 16
3 mCi in the target provided isolated (actual) yields of 16 3 mCi of
3 mCi of  At. A sixth step, used for purification of
At. A sixth step, used for purification of  At from trace metals, was evaluated in seven runs. In those runs, isolated
At from trace metals, was evaluated in seven runs. In those runs, isolated  At was distilled under reductive conditions to provide an average 71
At was distilled under reductive conditions to provide an average 71 8% recovery. RadioHPLC analyses of the isolated
8% recovery. RadioHPLC analyses of the isolated  At solutions, both initial and after distillation, were obtained to examine the
At solutions, both initial and after distillation, were obtained to examine the  At species present. The primary species of
At species present. The primary species of  At present was astatide, but astatate and unidentified species were also observed. Studies to determine the effect of bismuth attenuation on
At present was astatide, but astatate and unidentified species were also observed. Studies to determine the effect of bismuth attenuation on  At were conducted to estimate and attenuation factor (
At were conducted to estimate and attenuation factor ( 1.33) for adjustment of
1.33) for adjustment of  At readings in the bismuth target.
At readings in the bismuth target.
 At
At渡辺 茂樹; Gagnon, K.*; Hamlin, D. K.*; Chyan, M.-K.*; Balkin, E. R.*; Wilbur, D. S.*
no journal, ,
Difficulties with reproducibility of isolation yield when distilling  At from irradiated bismuth targets led us to use a "wet chemistry" approach for that process. The wet chemistry approach has provided
At from irradiated bismuth targets led us to use a "wet chemistry" approach for that process. The wet chemistry approach has provided  At isolation yields of 78% after decay and Bi attenuation corrections. However, the use of diisopropyl ether (DIPE) in the separation process has made it difficult to reach our goal of automating the
At isolation yields of 78% after decay and Bi attenuation corrections. However, the use of diisopropyl ether (DIPE) in the separation process has made it difficult to reach our goal of automating the  At reaction. Therefore, we have investigated the use of column materials to simplify the isolation of
At reaction. Therefore, we have investigated the use of column materials to simplify the isolation of  At and remove DIPE from the process. In this investigation, we evaluated the use of a strong anion exchange resin (AG1x8), a strong cation exchange resin (AG MP-50) and a polypropylene glycol (PEG)-coated resin for separation of
At and remove DIPE from the process. In this investigation, we evaluated the use of a strong anion exchange resin (AG1x8), a strong cation exchange resin (AG MP-50) and a polypropylene glycol (PEG)-coated resin for separation of  At from the bismuth target material.
At from the bismuth target material.
渡辺 茂樹; Balkin, E. R.*; Hamlin, D. K.*; Gagnon, K.*; Chyan, M.-K.*; Wilbur, D. S.*
no journal, ,
At-211(半減期:7.21時間)は、内用放射線治療への応用が期待されている 線放出核種である。At-211の製造では
線放出核種である。At-211の製造では He
He ビームを用いた
ビームを用いた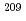 Bi(
Bi( ,2n)反応によるAt-211の生成、および、乾式蒸留法による分離が一般的に行われてきた。しかし、乾式蒸留法は回収率が安定しないという問題点に加えて、内用放射線治療での利用に必須となる装置化に適さない。そこで、安定した回収率が期待でき、かつ装置化に適した湿式分離法に着目し、本法を用いたBiターゲットからのAt-211の分離を実施した。本講演ではその成果を報告する。
,2n)反応によるAt-211の生成、および、乾式蒸留法による分離が一般的に行われてきた。しかし、乾式蒸留法は回収率が安定しないという問題点に加えて、内用放射線治療での利用に必須となる装置化に適さない。そこで、安定した回収率が期待でき、かつ装置化に適した湿式分離法に着目し、本法を用いたBiターゲットからのAt-211の分離を実施した。本講演ではその成果を報告する。
Wilbur, D. S.*; Hamlin, D. K.*; Chyan, M.-K.*; Gagnon, K.*; Balkin, E. R.*; 渡辺 茂樹
no journal, ,
Our desire to obtain higher and more consistent recovery of At-211 from large irradiated bismuth tagrets led to the refinement of a wet chemistry approach for isolation of At-211. The wet chemistry approach has provided very consistent isolation yields ( 75%) with no failures in over 200 irradiations. However, our desire to automate the process has been stymied by the miscibility and differential compressibility of the diisopropyl ether (DIPE) used in an extraction step in the process. As an alternative alkylether extractant to replace DIPE, we have investigated columns that contain polyethylene glycol (PEG) bound to a solid supoort.
75%) with no failures in over 200 irradiations. However, our desire to automate the process has been stymied by the miscibility and differential compressibility of the diisopropyl ether (DIPE) used in an extraction step in the process. As an alternative alkylether extractant to replace DIPE, we have investigated columns that contain polyethylene glycol (PEG) bound to a solid supoort.
渡辺 茂樹; 渡辺 智; Hamlin, D. K.*; Chyan, M.-K.*; Gagnon, K.*; 鈴木 博元*; Balkin, E. R.*; 大島 康宏; Wilbur, D. S.*; 石岡 典子
no journal, ,
アスタチン-211(At-211)は、RI内用療法への利用が期待される半減期7.2時間の 線放出RIである。本研究では、
線放出RIである。本研究では、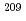 Bi(
Bi( ,2n)反応により生成したAt-211の分離について、乾式法および湿式法により検討した。乾式法では、加熱により気化させたAt-211をコールドトラップ後、種々の溶媒を通液することで回収した。溶媒として0.1M NaOH/エタノール=1/19(v/v)溶液を用いた結果、平均収率58%(n=24)で回収可能であった。一方、湿式法では、酸溶液中のAt-211がエーテルに効率よく抽出される点に着目し、ポリエチレングリコール樹脂を用いた分離を検討した。その結果、8M HClに溶解したAt-211は、99%でPEG樹脂に吸着され、15M NH
,2n)反応により生成したAt-211の分離について、乾式法および湿式法により検討した。乾式法では、加熱により気化させたAt-211をコールドトラップ後、種々の溶媒を通液することで回収した。溶媒として0.1M NaOH/エタノール=1/19(v/v)溶液を用いた結果、平均収率58%(n=24)で回収可能であった。一方、湿式法では、酸溶液中のAt-211がエーテルに効率よく抽出される点に着目し、ポリエチレングリコール樹脂を用いた分離を検討した。その結果、8M HClに溶解したAt-211は、99%でPEG樹脂に吸着され、15M NH OHで溶出した結果、収率79%でAt-211を回収できることを明らかにした。本発表では化学分離の結果に加えて、各分離法で得られるAt-211の化学形分析の結果も合わせて報告する予定である。
OHで溶出した結果、収率79%でAt-211を回収できることを明らかにした。本発表では化学分離の結果に加えて、各分離法で得られるAt-211の化学形分析の結果も合わせて報告する予定である。