Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ohashi, Yusuke; Ikeda, Yasuhisa*
Journal of Radioanalytical and Nuclear Chemistry, 321(2), p.683 - 691, 2019/08
Times Cited Count:6 Percentile:47.74(Chemistry, Analytical)Sediment waste generated from the waste solution of uranium handling facilities contains uranium and iron. In this study, we examined methods to selectively separate uranium from the waste without using nitric acid. Uranium dissolved in 0.5 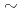 2.5M sulfuric acid, 1M hydrochloric acid, and 5M perchloric acid was selectively precipitated by adding oxalic acid. In addition, when oxalic acid and NCP are added together in 2.5M sulfuric acid, it seems that new insoluble uranium compounds are formed. Consequently, precipitation ratios of uranium was improved. Uranium was selectively precipitated from the sulfuric acid solution containing Fe and Al, and the precipitation ratio of uranium became 99%.
2.5M sulfuric acid, 1M hydrochloric acid, and 5M perchloric acid was selectively precipitated by adding oxalic acid. In addition, when oxalic acid and NCP are added together in 2.5M sulfuric acid, it seems that new insoluble uranium compounds are formed. Consequently, precipitation ratios of uranium was improved. Uranium was selectively precipitated from the sulfuric acid solution containing Fe and Al, and the precipitation ratio of uranium became 99%.
Ohashi, Yusuke; Tanaka, Yoshio; Tsunashima, Yasumichi; Ikeda, Yasuhisa*
Journal of Nuclear Science and Technology, 54(3), p.382 - 390, 2017/03
Times Cited Count:11 Percentile:67.55(Nuclear Science & Technology)Sludge-like uranium wastes (SUWs) have been generated with neutralization of acidic aqueous solutions used for decontamination of metal wastes containing a large amount of iron. We have examined the method for recovering uranium from such SUWs using 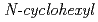 -2-pyrrolidone (NCP) as a precipitate. As a result, it was found that precipitation ratios (PRs) of uranium in the solutions prepared by dissolving SUWs in HNO
-2-pyrrolidone (NCP) as a precipitate. As a result, it was found that precipitation ratios (PRs) of uranium in the solutions prepared by dissolving SUWs in HNO is 97.7% at [NCP]/[U(VI)] = 20, and that the PRs of iron, aluminum, fluorine, and sulfate species are less than 1%. This indicates that uranium species are precipitated selectively. The content ratios of U, Fe, Ca, F, and S in the materials after calcining precipitates obtained at [NCP]/[U(VI)] = 20 were in accordance with the conditions of uranium ore concentrate. From these results, it is expected that highly purified uranium can be efficiently recovered from SUWs by using NCP as the precipitant.
is 97.7% at [NCP]/[U(VI)] = 20, and that the PRs of iron, aluminum, fluorine, and sulfate species are less than 1%. This indicates that uranium species are precipitated selectively. The content ratios of U, Fe, Ca, F, and S in the materials after calcining precipitates obtained at [NCP]/[U(VI)] = 20 were in accordance with the conditions of uranium ore concentrate. From these results, it is expected that highly purified uranium can be efficiently recovered from SUWs by using NCP as the precipitant.
Ohashi, Yusuke; Harada, Masayuki*; Asanuma, Noriko*; Ando, Shion; Tanaka, Yoshio; Ikeda, Yasuhisa*
Journal of Radioanalytical and Nuclear Chemistry, 311(1), p.491 - 502, 2017/01
Times Cited Count:1 Percentile:9.35(Chemistry, Analytical)In order to assess the feasibility of method for recovering U from wastes containing uranium (scrap uranium) using polyvinylpolypyrrolidone (PVPP) adsorbent, we have examined the adsorption and desorption behavior of metal species in HCl aqueous solutions dissolving scrap uranium. It was found that the U(VI) species are selectively adsorbed onto PVPP regardless of the presence of a large amount of Na(I) and Al(III), that the adsorbed U(VI) species are desorbed from PVPP column selectively by water. Pure uranium was efficiently recovered from the eluates. From these results, the PVPP resin is expected to be used as the adsorbent in the treatment process of scrap uranium.
Ohashi, Yusuke; Asanuma, Noriko*; Harada, Masayuki*; Tanaka, Yoshio; Ikeda, Yasuhisa*
Journal of Radioanalytical and Nuclear Chemistry, 309(2), p.627 - 636, 2016/08
Times Cited Count:8 Percentile:55.98(Chemistry, Analytical)As one of methods for recovering uranium from the uranium-bearing wastes, we have proposed the electrolytic deposition method using choline chloride-urea (CCU) which is known as an ambient temperature molten salt. More than 92% of uranium components in inactivated alumina and spent sodium fluoride adsorbent was dissolved into CCU solution. Cyclic voltammograms (CVs) of the solutions prepared by dissolving uranium-bearing wastes in CCU were measured in the potential range of -2.0 to 1.1 V (vs. Ag/AgCl). The one reduction peak was observed around -0.7 V for all solutions. Based on the results of CVs, bulk electrolyses of the solutions dissolving uranium-bearing wastes were also carried out at -1.5V at 80  C. The deposits were formed on a carbon electrode as cathode. Consequently, we confirmed that CCU is effective media for recovering uranium selectively from uranium-bearing waste.
C. The deposits were formed on a carbon electrode as cathode. Consequently, we confirmed that CCU is effective media for recovering uranium selectively from uranium-bearing waste.
Oshima, Katsumi; Oda, Yasuhisa; Takahashi, Koji; Terakado, Masayuki; Ikeda, Ryosuke; Hayashi, Kazuo*; Moriyama, Shinichi; Kajiwara, Ken; Sakamoto, Keishi
JAEA-Technology 2015-061, 65 Pages, 2016/03
In JAEA, an ITER relevant control system for ITER gyrotron was developed according to Plant Control Design Handbook. This control system was developed based on ITER CODAC Core System and implemented state machine control of gyrotron operation system, sequential timing control of gyrotron oscillation startup, and data acquisition. The operation of ITER 170 GHz gyrotron was demonstrated with ITER relevant power supply configuration. This system is utilized for gyrotron operation test for ITER procurement. This report describes the architecture of gyrotron operation system, its basic and detailed design, and recent operation results.
Ohashi, Yusuke; Harada, Masayuki*; Asanuma, Noriko*; Ikeda, Yasuhisa*
Journal of Nuclear Materials, 464, p.119 - 127, 2015/09
Times Cited Count:18 Percentile:79.54(Materials Science, Multidisciplinary)In order to examine feasibility of the electrochemical deposition method for recovering uranium from the solid wastes contaminated with uranium using ionic liquid as electrolyte, we have studied the electrochemical behavior of each solution prepared by soaking the spent NaF adsorbents and the steel waste contaminated with uranium in BMICl (1-butyl-3-methyl- imidazolium chloride). The uranyl(VI) species in BMICl solutions were found to be reduced to U(V) irreversibly around -0.8 to -1.3 V vs. Ag/AgCl. Based on the electrochemical data, we have performed potential controlled electrolysis of each solution at -1.5 V vs. Ag/AgCl. Black deposit was obtained, and their composition analyses suggest that the deposit is the mixtures of U(IV) and U(VI) compounds containing O, F, Cl, and N elements. From the present study, it is expected that the solid wastes contaminated with uranium can be decontaminated by treating them in BMICl and the dissolved uranium species are recovered electrolytically.
Suzuki, Tomoya; Takao, Koichiro*; Kawasaki, Takeshi*; Harada, Masayuki*; Nogami, Masanobu*; Ikeda, Yasuhisa*
Polyhedron, 96, p.102 - 106, 2015/08
Times Cited Count:8 Percentile:52.61(Chemistry, Inorganic & Nuclear)We have determined crystal structures of UO (NO
(NO )
) (
(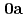 )
) (
(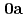 : 2-imidazolidone), UO
: 2-imidazolidone), UO (NO
(NO )
) (
(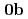 )
) (
(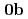 : tetrahydro-2-pyrimidone) and UO
: tetrahydro-2-pyrimidone) and UO (NO
(NO )
) (
(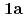 )
) (
(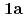 : 1-methyl-2-imidazolidone) by using single crystal X-ray analysis, and examined correlations between melting points (mps) and intermolecular hydrogen bonds (HBs) of UO
: 1-methyl-2-imidazolidone) by using single crystal X-ray analysis, and examined correlations between melting points (mps) and intermolecular hydrogen bonds (HBs) of UO (NO
(NO )
) (CU)
(CU) (CU: cyclic urea derivatives) and UO
(CU: cyclic urea derivatives) and UO (NO
(NO )
) (NRP)
(NRP) (NRP: pyrrolidone derivative).
(NRP: pyrrolidone derivative).
Kobayashi, Takayuki; Sawahata, Masayuki; Terakado, Masayuki; Hiranai, Shinichi; Ikeda, Ryosuke; Oda, Yasuhisa; Wada, Kenji; Hinata, Jun; Yokokura, Kenji; Hoshino, Katsumichi; et al.
Proceedings of 40th International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz 2015) (USB Flash Drive), 3 Pages, 2015/08
A gyrotron for electron cyclotron heating and current drive (ECH/CD) has been developed for JT-60SA (Super-Advanced). In high-power, long-pulse operations, oscillations of 1 MW/100 s have been demonstrated at both 110 GHz and 138 GHz, for the first time. These results fully satisfied the requirements for JT-60SA. Moreover, it was experimentally shown that the higher power operation at each frequency is expected to be acceptable for this gyrotron from the viewpoint of heat load at the cavity resonator, collector, and stray radiation absorbers. An oscillation at 82 GHz, which is an additional frequency, has been demonstrated up to 2 s at the output power of 0.4 MW, so far. High power experiments toward higher power of 1.5 MW (110/138 GHz) and 1 MW (82 GHz) are ongoing.
Kobayashi, Takayuki; Moriyama, Shinichi; Yokokura, Kenji; Sawahata, Masayuki; Terakado, Masayuki; Hiranai, Shinichi; Wada, Kenji; Sato, Yoshikatsu; Hinata, Jun; Hoshino, Katsumichi; et al.
Nuclear Fusion, 55(6), p.063008_1 - 063008_8, 2015/06
Times Cited Count:27 Percentile:75.46(Physics, Fluids & Plasmas)A gyrotron enabling high-power, long-pulse oscillations at both 110 GHz and 138 GHz has been developed for electron cyclotron heating (ECH) and current drive (CD) in JT-60SA. Oscillations of 1 MW for 100 s have been demonstrated at both frequencies, for the first time as a gyrotron operating at two frequencies. The optimization of the anode voltage, or the electron pitch factor, using a triode gun was a key to obtain high power and high efficiency at two frequencies. It was also confirmed that the internal losses in the gyrotron were sufficiently low for expected long pulse operation at the higher power level of  1.5 MW. Another important result is that an oscillation at 82 GHz, which enables to use fundamental harmonic waves in JT-60SA while the other two frequencies are used as second harmonics waves, was demonstrated up to 0.4 MW for 2 s. These results of the gyrotron development significantly contribute to enhancing operation regime of the ECH/CD system in JT-60SA.
1.5 MW. Another important result is that an oscillation at 82 GHz, which enables to use fundamental harmonic waves in JT-60SA while the other two frequencies are used as second harmonics waves, was demonstrated up to 0.4 MW for 2 s. These results of the gyrotron development significantly contribute to enhancing operation regime of the ECH/CD system in JT-60SA.
Sasaki, Yuji; Saeki, Morihisa; Sugo, Yumi; Ikeda, Yasuhisa*; Kawasaki, Takeshi*; Suzuki, Tomoya*; Ohashi, Akira*
Solvent Extraction Research and Development, Japan, 22(1), p.37 - 45, 2015/05
Times Cited Count:15 Percentile:42.12(Chemistry, Multidisciplinary)An extractant, methylimino-bis- -dioctylacetamide (MIDOA), was used for the extraction of soft acid metals. It was found that MIDOA can extract not only Cr(VI), Mo(VI), W(VI), Tc(VII) and Re(VII), whose metals can form the oxonium anions due to their high oxidation states, but also other metal cations, like Nb(V), Ta(V) and Pd(II). Analogous compounds, imino-bis-
-dioctylacetamide (MIDOA), was used for the extraction of soft acid metals. It was found that MIDOA can extract not only Cr(VI), Mo(VI), W(VI), Tc(VII) and Re(VII), whose metals can form the oxonium anions due to their high oxidation states, but also other metal cations, like Nb(V), Ta(V) and Pd(II). Analogous compounds, imino-bis- -dioctylacetamide (IDOA) and methylimino-bis-
-dioctylacetamide (IDOA) and methylimino-bis- -di-2-ethylhexylacetamide (MIDEHA), are synthesized and compared for their extractability. It is clear that these extractants have almost same or lower
-di-2-ethylhexylacetamide (MIDEHA), are synthesized and compared for their extractability. It is clear that these extractants have almost same or lower  values than those for MIDOA. In order to examine the effect on extractability with different donor atoms, TODGA (
values than those for MIDOA. In order to examine the effect on extractability with different donor atoms, TODGA ( -tetraoctyl-diglycolamide) and TDGA (
-tetraoctyl-diglycolamide) and TDGA ( -tetraoctyl-tyiodiglycolamide) having oxygen and sulfur donor are employed. The comparison of their extractabilities suggests that the trend of Pd and Re extraction is N
-tetraoctyl-tyiodiglycolamide) having oxygen and sulfur donor are employed. The comparison of their extractabilities suggests that the trend of Pd and Re extraction is N  S
S  O and N
O and N  O
O  S, respectively.
S, respectively.
 -alkylated 2-pyrrolidone derivatives
-alkylated 2-pyrrolidone derivativesTakao, Koichiro*; Kawata, Yoshihisa*; Nogami, Masanobu*; Harada, Masayuki*; Morita, Yasuji; Nishimura, Kenji*; Ikeda, Yasuhisa*
Journal of Nuclear Science and Technology, 52(2), p.294 - 298, 2015/02
Times Cited Count:2 Percentile:16.17(Nuclear Science & Technology)Yields of precipitated UO (NO
(NO )
) (NRP)
(NRP) (NRP =
(NRP =  -alkylated 2-pyrrolidone) were precisely determined by considering reduction of the solution volume through the precipitation, which can be estimated from difference in acid concentrations of the liquid phases before and after the precipitation. The studied NRPs were
-alkylated 2-pyrrolidone) were precisely determined by considering reduction of the solution volume through the precipitation, which can be estimated from difference in acid concentrations of the liquid phases before and after the precipitation. The studied NRPs were  -
- -butyl (NBP) and
-butyl (NBP) and  -
- -propyl (NProP) derivatives. In both systems, the precipitation yields precisely determined were always higher than those simply calculated from the ratio of uranium concentrations before and after the precipitation. However, the differences between them are in the range of 0.6% - 2.6%. If such a difference is practically negligible, the volume reduction through the precipitation does not have to be taken into account for simplicity of the analytical manipulation.
-propyl (NProP) derivatives. In both systems, the precipitation yields precisely determined were always higher than those simply calculated from the ratio of uranium concentrations before and after the precipitation. However, the differences between them are in the range of 0.6% - 2.6%. If such a difference is practically negligible, the volume reduction through the precipitation does not have to be taken into account for simplicity of the analytical manipulation.
Oda, Yasuhisa; Oshima, Katsumi; Nakamoto, Takashi*; Hashimoto, Yasunori*; Yamamoto, Tsuyoshi; Hayashi, Kazuo*; Ikeda, Yukiharu; Ikeda, Ryosuke; Kajiwara, Ken; Takahashi, Koji; et al.
Purazuma, Kaku Yugo Gakkai-Shi, 90(7), p.365 - 373, 2014/07
no abstracts in English
Sasaki, Yuji; Tsubata, Yasuhiro; Kitatsuji, Yoshihiro; Sugo, Yumi; Shirasu, Noriko; Ikeda, Yasuhisa*; Kawasaki, Takeshi*; Suzuki, Tomoya*; Mimura, Hitoshi*; Usuda, Shigekazu*; et al.
JAEA-Research 2014-008, 220 Pages, 2014/06
The researches on Development of mutual separation technology of minor actinides by the novel hydrophilic and lipophilic diamide compounds, entrusted to Japan Atomic Energy Agency by the Ministry of Education, Culture, Sports, Science and Technology of Japan, from 2010 to 2012 are summarized. This project was composed of three themes, those are (1) Development of total recovery of MA+Ln: basic researches for new extractant, DOODA, (2) Development of mutual separation of Am/Cm/Ln: basic researches of Ln-complex, solvent extraction, and extraction chromatography, and (3) Evaluation of separation technique: process simulation. For topic (1), we summarized the information on characteristic of DOODA extractant. For topic (2), we summarized the information on structures of Ln-complexes, solvent extraction and chromatography. For topic (3), we summarized the information on conditions of mixer-settler and evaluation of each fraction separated.
 ]) solution of [EMI]
]) solution of [EMI] [UO
[UO (NO
(NO )
) ]; First spectrophotometric evidence for existence of [UO
]; First spectrophotometric evidence for existence of [UO (NO
(NO )
) ]
]
Sasaki, Kotoe*; Suzuki, Tomoya*; Arai, Tsuyoshi*; Takao, Koichiro*; Suzuki, Shinichi; Yaita, Tsuyoshi; Ikeda, Yasuhisa*
Chemistry Letters, 43(5), p.670 - 672, 2014/05
Times Cited Count:8 Percentile:27.41(Chemistry, Multidisciplinary)no abstracts in English
Ohashi, Yusuke; Nomura, Mitsuo; Tsunashima, Yasumichi; Ando, Shion; Sugitsue, Noritake; Ikeda, Yasuhisa*; Tanaka, Yoshio
Journal of Nuclear Science and Technology, 51(2), p.251 - 265, 2014/02
Times Cited Count:10 Percentile:57.73(Nuclear Science & Technology)Sludge-like uranium-bearing wastes generated from uranium refining and conversion R&D facilities are stored at the Ningyo-toge Environmental Engineering Center. We have proposed an aqueous process for recovering uranium from spent filter aid and CaF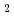 precipitate using hydrochloric acid. The distributions of the dissolved species in the sample solutions at different pH levels were calculated using the chemical equilibrium modeling system. Calculated results of fluorine contents of recovered uranium were compared with the experimental results. The fluorine content in the recovered uranium decreased as the aluminum concentration of the solution increased. On the other hand, uranium of spent filter aid was recovered selectively. The size of the particles of recovered uranium tends to decrease with increasing pH in the precipitation treatments. Also, the uranium concentration of the precipitate generated by the neutralization of the barren solution falls below 1 Bq/g.
precipitate using hydrochloric acid. The distributions of the dissolved species in the sample solutions at different pH levels were calculated using the chemical equilibrium modeling system. Calculated results of fluorine contents of recovered uranium were compared with the experimental results. The fluorine content in the recovered uranium decreased as the aluminum concentration of the solution increased. On the other hand, uranium of spent filter aid was recovered selectively. The size of the particles of recovered uranium tends to decrease with increasing pH in the precipitation treatments. Also, the uranium concentration of the precipitate generated by the neutralization of the barren solution falls below 1 Bq/g.
 -ray irradiation in HNO
-ray irradiation in HNO media
mediaNogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Kawata, Yoshihisa*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Journal of Radioanalytical and Nuclear Chemistry, 296(1), p.423 - 427, 2013/04
Times Cited Count:6 Percentile:41.67(Chemistry, Analytical)Stability of polyvinylpolypyrrolidone (PVPP), a resin with adsorption selectivity to U(VI) in nitric acid media, against  -ray irradiation has been examined using HNO
-ray irradiation has been examined using HNO solutions of various concentrations. As the result, no clear decrease in the capacity was observed for any samples. Or rather, it was found that the capacity increased by approximately 50% for the PVPP slurry irradiated in 6 M HNO
solutions of various concentrations. As the result, no clear decrease in the capacity was observed for any samples. Or rather, it was found that the capacity increased by approximately 50% for the PVPP slurry irradiated in 6 M HNO . The infrared spectroscopic study indicates that PVPP degrades by
. The infrared spectroscopic study indicates that PVPP degrades by  -ray irradiation in HNO
-ray irradiation in HNO from the cleavage of the pyrrolidone ring by the addition of oxygen atom originating from HNO
from the cleavage of the pyrrolidone ring by the addition of oxygen atom originating from HNO , followed by the formation of chain monoamides with multiple coordinative atoms by the continuous addition of oxygen, finally leading to the generation of primary-amine type anion exchange resin. It is also indicated that all generated functional groups possess adsorptivity to U(VI) in 3 M HNO
, followed by the formation of chain monoamides with multiple coordinative atoms by the continuous addition of oxygen, finally leading to the generation of primary-amine type anion exchange resin. It is also indicated that all generated functional groups possess adsorptivity to U(VI) in 3 M HNO .
.
 -ray irradiation
-ray irradiationNogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Kawata, Yoshihisa*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Science China; Chemistry, 55(9), p.1739 - 1745, 2012/09
Times Cited Count:4 Percentile:19.06(Chemistry, Multidisciplinary)Stability of N-alkylated pyrrolidone derivatives (NRPs) against radiation was examined by irradiation tests with  Co
Co  -ray. We have been developed a novel reprocessing system using NRPs which have precipitation ability to haxa- and tetravalent actinides in nitric acid media. Degradation rates of NRPs are evaluated by irradiation in 3M nitric acid solutions and mechanism of degradation are discussed in the present paper.
-ray. We have been developed a novel reprocessing system using NRPs which have precipitation ability to haxa- and tetravalent actinides in nitric acid media. Degradation rates of NRPs are evaluated by irradiation in 3M nitric acid solutions and mechanism of degradation are discussed in the present paper.
Minato, Kazuo; Konashi, Kenji*; Yamana, Hajimu*; Yamanaka, Shinsuke*; Nagasaki, Shinya*; Ikeda, Yasuhisa*; Sato, Seichi*; Arita, Yuji*; Idemitsu, Kazuya*; Koyama, Tadafumi*
Proceedings of International Conference on Toward and Over the Fukushima Daiichi Accident (GLOBAL 2011) (CD-ROM), 5 Pages, 2011/12
Actinide science research is indispensable to maintain sustainable development of innovative nuclear technology. For actinide science research, special facilities with containment and radiation shields are needed to handle actinide materials. The number of facilities for actinide science research has been decreased, especially in universities, due to the high maintenance cost. J-ACTINET was established in 2008 to promote and facilitate actinide science research and to foster many of young scientists and engineers in actinide science. The research program was carried out, through which young researchers were expected to learn how to make experiments with advanced experimental tools and to broaden their horizons. The summer schools and computational science school were held to provide students and young researchers with the opportunities to come into contact with actinide science research. The overseas dispatch program was also carried out.
Ikeda, Yasuhisa*; Kawasaki, Takeshi*; Harada, Masayuki*; Nogami, Masanobu*; Kawata, Yoshihisa*; Kim, S.-Y.*; Morita, Yasuji; Chikazawa, Takahiro*; Someya, Hiroshi*; Kikuchi, Toshiaki*
Proceedings of International Conference on Toward and Over the Fukushima Daiichi Accident (GLOBAL 2011) (CD-ROM), 5 Pages, 2011/12
An advanced reprocessing system for spent FBR fuels based on two precipitation processes using pyrrolidone derivatives as precipitants has been developed. Experimental results of precipitation behavior of U, Pu and other elements, the heat- and radiation-resistance of precipitants, the thermal decomposition properties of precipitates showed that N-n-butyl-2-pyrrolidone and N-neopentyl-2-pyrrolidone are the appropriate precipitants for the first and second precipitation steps, respectively. From the engineering investigation, We confirmed that the precipitation and the filtration can be done efficiently using the engineering scale equipment and that the fuel pellets are directly prepared by the calcination of the precipitates. On the basis of these results, we evaluated that the proposed system is expected to be one of candidates of the future reprocessing systems for spent FBR fuels.
Nogami, Masanobu*; Harada, Masayuki*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Kawata, Yoshihisa*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Progress in Nuclear Energy, 53(7), p.948 - 951, 2011/09
Times Cited Count:4 Percentile:31.26(Nuclear Science & Technology)The precipitation ability of 1,3-dimethyl-2-imidazolidone (DMI) to U(VI) and U(IV) was examined using nitric acid solutions. While DMI precipitated U(VI) from 3 M nitric acid, no precipitate was observed in the solution containing 0.15 M U(IV) and 3 M nitric acid by adding DMI at the ratio of [DMI]/[U(IV)]=5. This indicates that the selectivity of DMI to U(VI) than U(IV). In spite of the excellent selectivity to U(VI), DMI has a disadvantage on the stability in nitric acid, because gradual acid hydrolysis of DMI is inevitable due to the nature of the chemical structure. Experiments on the stability of DMI in  -ray irradiation and heating in nitric acid solutions showed that the stability is strongly affected by the concentration of nitric acid and that DMI may be applicable in nitric acid solutions up to ca. 2 M.
-ray irradiation and heating in nitric acid solutions showed that the stability is strongly affected by the concentration of nitric acid and that DMI may be applicable in nitric acid solutions up to ca. 2 M.