Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Aoyama, Takahito; Ueno, Fumiyoshi; Sato, Tomonori; Kato, Chiaki; Sano, Naruto; Yamashita, Naoki; Otani, Kyohei; Igarashi, Takahiro
Annals of Nuclear Energy, 214, p.111229_1 - 111229_6, 2025/05
Times Cited Count:0 Percentile:0.00(Nuclear Science & Technology)Irisawa, Eriko; Kato, Chiaki
Journal of Nuclear Materials, 591, p.154914_1 - 154914_10, 2024/04
Times Cited Count:4 Percentile:86.15(Materials Science, Multidisciplinary)The amount of corrosion of austenitic stainless-steel R-SUS304ULC was evaluated considering the changes in solution composition and boiling during actual concentration operations. Austenitic stainless-steel R-SUS304ULC is the structural material of the highly radioactive liquid waste concentrator in Japanese spent fuel reprocessing plant, which treats highly corrosive nitric acid solutions during enrichment operations. The study results show that it is necessary to focus on nitric acid concentrations, oxidizing metal ion concentrations, and decompression boiling as factors that accelerate the corrosion rate of stainless steel because of cathodic reaction activation.
Sato, Tomonori; Hata, Kuniki; Kato, Chiaki; Igarashi, Takahiro
Zairyo To Kankyo, 73(4), p.102 - 109, 2024/04
To evaluate the effects of dissolved oxygen concentration to water quality within SCC crack and the distribution of water quality in the depth direction under irradiation, immersion tests of stainless steel specimens given a gap and water radiolysis calculations for the water quality in the crevice gap were performed. As a result, it was confirmed that Fe O
O was formed in the entire area within the crevice regardless of the dissolved oxygen concentration. It was also estimated that under irradiation, the oxidant species produced directly by radiolysis in the crack are consumed by the oxide growth, and anion enrichment occurs in the crack even in the irradiation conditions.
was formed in the entire area within the crevice regardless of the dissolved oxygen concentration. It was also estimated that under irradiation, the oxidant species produced directly by radiolysis in the crack are consumed by the oxide growth, and anion enrichment occurs in the crack even in the irradiation conditions.
Yamashita, Naoki; Aoyama, Takahito; Kato, Chiaki; Sano, Naruto; Tagami, Susumu
JAEA-Technology 2023-028, 22 Pages, 2024/03
At the Fukushima Daiichi Nuclear Power Station (1F), which is currently undergoing decommissioning, there is growing interest in the effects of radiation-emitting radionuclides such as  Sr and
Sr and  Cs on the structural integrity. In particular, the corrosion behavior of carbon steel, which is used in many parts of 1F, is known to change depending on metal cations in solution, but the effects of
Cs on the structural integrity. In particular, the corrosion behavior of carbon steel, which is used in many parts of 1F, is known to change depending on metal cations in solution, but the effects of  Sr and
Sr and  Cs on corrosion are not yet understood. In addition, it is important to investigate the distribution of
Cs on corrosion are not yet understood. In addition, it is important to investigate the distribution of  Sr and
Sr and  Cs in the rust layer in order to understand the corrosion behavior, but the method has not yet been established. In this study, a glove box was prepared to conduct corrosion tests of carbon steel in NaCl containing
Cs in the rust layer in order to understand the corrosion behavior, but the method has not yet been established. In this study, a glove box was prepared to conduct corrosion tests of carbon steel in NaCl containing  Sr and
Sr and  Cs in the glove box. In addition, in order to clarify the influence of
Cs in the glove box. In addition, in order to clarify the influence of  Sr and
Sr and  Cs, which exist as metal cations in the solution, on the corrosion behavior of carbon steel, we attempted to establish a detection method for radioactive materials in the rust layer using an imaging plate.
Cs, which exist as metal cations in the solution, on the corrosion behavior of carbon steel, we attempted to establish a detection method for radioactive materials in the rust layer using an imaging plate.
Otani, Kyohei; Kato, Chiaki; Igarashi, Takahiro
Corrosion, 79(11), p.1277 - 1286, 2023/11
 Sr and
Sr and  Cs in HCl solutions on the corrosion potential of Type 316L stainless steel
Cs in HCl solutions on the corrosion potential of Type 316L stainless steelAoyama, Takahito; Sato, Tomonori; Ueno, Fumiyoshi; Kato, Chiaki; Sano, Naruto; Yamashita, Naoki; Igarashi, Takahiro
Zairyo To Kankyo, 72(11), p.284 - 288, 2023/11
no abstracts in English
Hata, Kuniki; Kimura, Atsushi*; Taguchi, Mitsumasa*; Sato, Tomonori; Kato, Chiaki; Watanabe, Yutaka*
Zairyo To Kankyo, 72(4), p.126 - 130, 2023/04
Gamma-radiolysis experiments with gas-liquid coexistent samples were carried out to investigate effects of gas-phase radiolysis on corrosive environment for materials in solutions under irradiation. After gamma-ray irradiation, hydrogen peroxide, nitrate ion, nitrite ion were detected in the liquid phase. The production yields of nitrate ion and nitrite ion increased with increasing gas-phase volume and oxygen concentration. This result indicated that chemical reactions including oxygen and nitrogen in the gas phase were required for the production of nitrate ion and nitrite ion. To magnify the effects of gas-phase radiolysis in the gas-liquid coexistent samples, absorption dose rate in the liquid phase was reduced by one-hundredth using lead shield. The concentration of hydrogen peroxide and the pH in the shielded liquid phase were similar to those in the irradiated pure water, which did not contact with gas phase. This result indicated that the effects of nitrate ion and nitrite ion dissolved in the liquid phase on water radiolysis were not important in the current experimental system, in which the effects of gas-phase radiolysis were increased by 100-times.
Yamashita, Naoki; Irisawa, Eriko; Kato, Chiaki; Sano, Naruto; Tagami, Susumu
JAEA-Technology 2022-035, 29 Pages, 2023/03
In the treatment process of the current commercial reprocessing plant (Rokkasho Reprocessing Plant), the high-level liquid waste concentrator is the equipment that treats the most corrosive solution. In the high-level liquid waste concentrator, the extracted liquid waste after separation of uranium and plutonium is heated, concentrated, and reduced in volume. Therefore, the amount of gamma- rays emitted from fission products and the concentration of corrosive metal ion species such as neptunium-237 ( Np) are the highest in the reprocessing process, and the amount of corrosion in the high-level liquid waste concentrate canner is expected to be large. In this study, in order to clarify the effect of gamma-rays on the corrosion reaction of stainless steel in nitric acid solutions containing
Np) are the highest in the reprocessing process, and the amount of corrosion in the high-level liquid waste concentrate canner is expected to be large. In this study, in order to clarify the effect of gamma-rays on the corrosion reaction of stainless steel in nitric acid solutions containing  Np from the electrochemical viewpoint, the corrosion test apparatus for heat transfer surfaces in an airtight concrete cell at the Waste Safety TEsting Facility (WASTEF) of Nuclear Science Research Institute was modified to enable electrochemical measurements under gamma-ray irradiation. The effect of gamma-rays on the corrosion reaction taking place on the stainless steel surface was discussed from the electrochemical test results obtained. As a result, changes in the immersion potentials of stainless steel and the polarization curves due to chemical species caused by radiolysis of gamma-ray irradiation were confirmed.
Np from the electrochemical viewpoint, the corrosion test apparatus for heat transfer surfaces in an airtight concrete cell at the Waste Safety TEsting Facility (WASTEF) of Nuclear Science Research Institute was modified to enable electrochemical measurements under gamma-ray irradiation. The effect of gamma-rays on the corrosion reaction taking place on the stainless steel surface was discussed from the electrochemical test results obtained. As a result, changes in the immersion potentials of stainless steel and the polarization curves due to chemical species caused by radiolysis of gamma-ray irradiation were confirmed.
 to the inside of the crevice by chelation and its effect on crevice corrosion of Type 316L stainless steel
to the inside of the crevice by chelation and its effect on crevice corrosion of Type 316L stainless steelAoyama, Takahito; Kato, Chiaki
Corrosion Science, 210(2), p.110850_1 - 110850_10, 2023/01
Times Cited Count:7 Percentile:43.24(Materials Science, Multidisciplinary)The chelated complex of Cu : [Cu(EDTA)]
: [Cu(EDTA)] was used to introduce Cu
was used to introduce Cu from outside to the inside of crevice. The introduced Cu
from outside to the inside of crevice. The introduced Cu was expected to act as an inhibitor for the crevice corrosion on stainless steels. Crevice corrosion tests, confirmed the introduction of Cu
was expected to act as an inhibitor for the crevice corrosion on stainless steels. Crevice corrosion tests, confirmed the introduction of Cu to the inside of the crevice via electromigration of [Cu(EDTA)]
to the inside of the crevice via electromigration of [Cu(EDTA)] was confirmed. Migrated [Cu(EDTA)]
was confirmed. Migrated [Cu(EDTA)] reacted with H
reacted with H and inhibited decrease in pH inside the crevice, where Cu
and inhibited decrease in pH inside the crevice, where Cu was separated from [Cu(EDTA)]
was separated from [Cu(EDTA)] and suppressed active dissolution of the stainless steel.
and suppressed active dissolution of the stainless steel.
Soma, Yasutaka; Kato, Chiaki
Zairyo To Kankyo 2022 Koenshu (CD-ROM), p.219 - 220, 2022/05
It is important to understand the electrochemical properties of stainless steel in environment created within crevice of stainless steel in high temperature water (crevice environment). This is because acidification and concentration of impurity ions occur in the crevice environment and this is common inside the stress corrosion crack. In this study, we reproduced the crevice environment in bulk scale and investigated mainly the effect of Cr concentration on the electrochemical properties of Fe-Cr-Ni alloys. Polarization curves of Fe-20Ni-xCr (x=16.4, 23, 26) were measured in water with a temperature of 288 C, a Cl concentration of 2
C, a Cl concentration of 2 10
10 mol/dm
mol/dm , a pH value of about 4.5, and a dissolved hydrogen concentration of 10 ppb. The peak currents of active dissolution (at -400 mV) and passive current density (at -50 mV) for specimens with Cr concentrations x = 16.4, 23, and 26% were approximately 13.8, 15.9, 10.0
, a pH value of about 4.5, and a dissolved hydrogen concentration of 10 ppb. The peak currents of active dissolution (at -400 mV) and passive current density (at -50 mV) for specimens with Cr concentrations x = 16.4, 23, and 26% were approximately 13.8, 15.9, 10.0  Acm
Acm , and 18.4, 8.5, 8.5
, and 18.4, 8.5, 8.5  Acm
Acm , respectively. Although the current values of x=26 were slightly lower in both cases, it was concluded that there was no clear dependence of the polarization curve on Cr concentration in this environment.
, respectively. Although the current values of x=26 were slightly lower in both cases, it was concluded that there was no clear dependence of the polarization curve on Cr concentration in this environment.
Igarashi, Takahiro; Otani, Kyohei; Komatsu, Atsushi; Kato, Chiaki; Sakairi, Masatoshi*
Bosei Kanri, 66(4), p.141 - 145, 2022/04
Metal corrosion is a material deterioration phenomenon based on electrochemical reactions on an atomic scale. In this paper, various methods for acquiring physical properties on metal surfaces using first-principles calculations were described. As examples of applying first-principles calculation to metal corrosion, the effect of hydrogen adsorption on the metal surface on the potential change and the effect of cation atoms in the aqueous solution on the corrosion resistance of the metal were reported.
 Sr dissolved solution on corrosion potential of type 316L stainless steel
Sr dissolved solution on corrosion potential of type 316L stainless steelAoyama, Takahito; Kato, Chiaki; Sato, Tomonori; Sano, Naruto; Yamashita, Naoki; Ueno, Fumiyoshi
Zairyo To Kankyo, 71(4), p.110 - 115, 2022/04
no abstracts in English
 Np
NpIrisawa, Eriko; Kato, Chiaki; Yamashita, Naoki; Sano, Naruto
Zairyo To Kankyo, 71(3), p.70 - 74, 2022/03
In order to evaluate the corrosion of stainless steels used in spent nuclear fuel reprocessing facilities, the immersion corrosion tests and polarization measurements were performed using R-SUS304ULC stainless steel in nitric acid solution containing a kind of radionuclides,  Np. At temperatures above 328 K, the corrosion potential was higher than that in nitric acid solution and was near the transpassive region. From the comparison between the corrosion amount calculated by the immersion corrosion tests and the polarization resistance, the values of
Np. At temperatures above 328 K, the corrosion potential was higher than that in nitric acid solution and was near the transpassive region. From the comparison between the corrosion amount calculated by the immersion corrosion tests and the polarization resistance, the values of 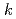 =0.018-0.025 V were obtained as a conversion factor, and the possibility of calculating the corrosion amount from the electrochemical measurement was examined.
=0.018-0.025 V were obtained as a conversion factor, and the possibility of calculating the corrosion amount from the electrochemical measurement was examined.
Otani, Kyohei; Ueno, Fumiyoshi; Kato, Chiaki
Zairyo To Kankyo, 71(2), p.40 - 45, 2022/02
The purpose of this study is to investigate the effect of oxygen concentration in the air on the corrosion rate of carbon steel in an air/solution alternating environment in the low oxygen concentration range and to clarify the corrosion rate and corrosion mechanism of carbon steel depending on the oxygen concentration in air by the mass change of specimens before and after the corrosion test and observing the iron rust layer formed on the surface of carbon steel. The corrosion rate increases with increasing oxygen concentration in the air, and the gradient of the corrosion rate decreases gradually. The maximum erosion depth increased with increasing oxygen concentration except for the case of 1% oxygen concentration, however, the maximum erosion depth for 1% oxygen concentration was larger than that for 5% air oxygen concentration.
Igarashi, Takahiro; Komatsu, Atsushi; Kato, Chiaki; Sakairi, Masatoshi*
Tetsu To Hagane, 107(12), p.998 - 1003, 2021/12
Times Cited Count:1 Percentile:4.80(Metallurgy & Metallurgical Engineering)In order to clarify the effect of environmental factors on the amount of atmospheric corrosion of steel, novel model for predicting the reduction of atmospheric corrosion considering relative humidity and rain falls was developed. We conducted a one-year calculation simulation of atmospheric corrosion in Miyakojima City, Choshi City, and Tsukuba City using the developed model. Corrosion weight loss by the simulation could reproduce the measured value well. Corrosion weight loss at each point was greatly affected by the amount of flying sea salt, relative humidity, and rain falls.
Otani, Kyohei; Kato, Chiaki
Zairyo To Kankyo, 70(12), p.480 - 486, 2021/12
This is a comprehensive paper of the corrosion of carbon steel in air/solution alternating condition. From cross-sectional observation and analysis of the iron rust layer formed on the surface of carbon steel in the alternating condition, it was found that a multilayered iron rust layer composed of red rust layer ( -FeOOH), rust crust layer (Fe
-FeOOH), rust crust layer (Fe O
O ), inner crystal (Fe
), inner crystal (Fe O
O ), and inner rust layer was formed on carbon steel. The multi-layered iron rust layer would accelerate the cathodic oxygen reduction reaction, and the reason why the corrosion rate of the carbon steel in the alternating condition was accelerated. The effect of artificial seawater (ASW) composition on the corrosion rate of carbon steel in air/solution alternating condition was investigated. It was found that the corrosion rate increased with increasing concentration from pure water to 200 times diluted ASW, and decreased with increasing concentration from 20 times diluted ASW to no diluted ASW. The Mg and Ca ions in ASW precipitated on the reaction interface and formed a metal cation layer, which inhibited the oxygen reduction reaction, and thus the corrosion of carbon steel was inhibited in the highly concentrated ASW.
), and inner rust layer was formed on carbon steel. The multi-layered iron rust layer would accelerate the cathodic oxygen reduction reaction, and the reason why the corrosion rate of the carbon steel in the alternating condition was accelerated. The effect of artificial seawater (ASW) composition on the corrosion rate of carbon steel in air/solution alternating condition was investigated. It was found that the corrosion rate increased with increasing concentration from pure water to 200 times diluted ASW, and decreased with increasing concentration from 20 times diluted ASW to no diluted ASW. The Mg and Ca ions in ASW precipitated on the reaction interface and formed a metal cation layer, which inhibited the oxygen reduction reaction, and thus the corrosion of carbon steel was inhibited in the highly concentrated ASW.
Kato, Chiaki; Yamagishi, Isao; Sato, Tomonori; Yamamoto, Masahiro*
Zairyo To Kankyo, 70(12), p.441 - 447, 2021/12
Zeolite particles have been used in a Cs adsorption vessel for purification of contaminated water in Fukushima Daiichi Nuclear Power Station (1F). The used Cs adsorption vessels were kept in storage space on 1F site. The risk of localized corrosion of stainless steel used in the vessel was worried. To evaluate the risk of localized corrosion, using specially designed electrochemical testing apparatus was used under gamma-ray irradiation test. And, real size mock-up test conducted. The results showed the potential change caused by creation of H O
O by water radiolysis decreased by zeolite particles and the enrichment of chloride ion concentration in the vessel do not propagate during dry up procedure of Cs adsorption vessel. These data indicate the risk of localized corrosion of Cs adsorption vessel may stay at considerably low level.
by water radiolysis decreased by zeolite particles and the enrichment of chloride ion concentration in the vessel do not propagate during dry up procedure of Cs adsorption vessel. These data indicate the risk of localized corrosion of Cs adsorption vessel may stay at considerably low level.
Igarashi, Takahiro; Komatsu, Atsushi; Kato, Chiaki; Sakairi, Masatoshi*
Bosei Kanri, 65(10), p.365 - 370, 2021/10
We have developed a new atmospheric simulation model considering important environmental factors such as airborne sea salt, temperature, relative humidity, and rainfall. The developed model was verified by comparing predicted values by the simulation and measured data for the weight loss by atmospheric corrosion. In addition, atmospheric corrosion simulations under open and sheltered exposure condition were conducted, and it was confirmed that the air corrosion weight loss was strongly suppressed by the surface cleaning effect due to rainfall.
Soma, Yasutaka; Kato, Chiaki
Dai-68-Kai Zairyo To Kankyo Toronkai Koenshu (CD-ROM), p.205 - 206, 2021/10
This study investigates the effect of temperature on dissipation behavior of Cl ion within the crevice of stainless steel. Concentration of Cl ion was evaluated by conductivity measured by using sensors installed at crevice specimen. At 50 and 80  C, Cl ions within the crevice of PEEK and Pt dissipated in accordance with concentration diffusion. On the contrary, dissipation speed of Cl ions inside the Type-304L stainless steel were much lower than those anticipated by simple concentration diffusion. This behavior attribute to the anodic dissolution of stainless steel inside the crevice, therefore, to quantitatively understand the effect of temperature on the dissipation behavior, it is necessary to know the anodic dissolution rate and occurrence of localized corrosion. Numerical analysis taking the effect of concentration diffusion and migration into account is also needed.
C, Cl ions within the crevice of PEEK and Pt dissipated in accordance with concentration diffusion. On the contrary, dissipation speed of Cl ions inside the Type-304L stainless steel were much lower than those anticipated by simple concentration diffusion. This behavior attribute to the anodic dissolution of stainless steel inside the crevice, therefore, to quantitatively understand the effect of temperature on the dissipation behavior, it is necessary to know the anodic dissolution rate and occurrence of localized corrosion. Numerical analysis taking the effect of concentration diffusion and migration into account is also needed.
Igarashi, Takahiro; Otani, Kyohei; Kato, Chiaki; Sakairi, Masatoshi*; Togashi, Yusuke*; Baba, Kazuhiko*; Takagi, Shusaku*
ISIJ International, 61(4), p.1085 - 1090, 2021/04
Times Cited Count:2 Percentile:10.12(Metallurgy & Metallurgical Engineering)In order to clarify the effect of metal cations (Zn , Mg
, Mg , Na
, Na ) in aqueous solution on hydrogen permeation into iron, the amount of hydrogen permeation from iron surface was measured by electrochemical tests with a laser ablation. Moreover, in order to obtain the basic mechanism of hydrogen permeation with metal cation, first-principles calculations were used to acquire the adsorption potential of the metal cation and the electronic state around iron surface. By Zn
) in aqueous solution on hydrogen permeation into iron, the amount of hydrogen permeation from iron surface was measured by electrochemical tests with a laser ablation. Moreover, in order to obtain the basic mechanism of hydrogen permeation with metal cation, first-principles calculations were used to acquire the adsorption potential of the metal cation and the electronic state around iron surface. By Zn in solution, anodic reaction on ablated surface by laser irradiation was suppressed. Also, by quantum analysis Zn atoms were chemically bonded stronger than Na and Mg atoms to iron surface. It was suggested that the dissolution reaction of iron was suppressed by the formation of the Zn layer, and that lead suppression of hydrogen permeation into iron.
in solution, anodic reaction on ablated surface by laser irradiation was suppressed. Also, by quantum analysis Zn atoms were chemically bonded stronger than Na and Mg atoms to iron surface. It was suggested that the dissolution reaction of iron was suppressed by the formation of the Zn layer, and that lead suppression of hydrogen permeation into iron.