Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 (
(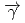 ,
,
 )
)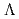 and
and  (
(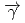 ,
,
 )
)
 reactions for
reactions for 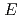
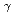 =1.5-2.4 GeV
=1.5-2.4 GeVZegers, R. G. T.*; Sumihama, Mizuki*; Ahn, D. S.*; Ahn, J. K.*; Akimune, Hidetoshi*; Asano, Yoshihiro; Chang, W. C.*; Dat , S.*; Ejiri, Hiroyasu*; Fujimura, Hisako*; et al.
, S.*; Ejiri, Hiroyasu*; Fujimura, Hisako*; et al.
Physical Review Letters, 91(9), p.092001_1 - 092001_4, 2003/08
Times Cited Count:128 Percentile:94.88(Physics, Multidisciplinary)no abstracts in English
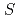 = +1 Baryon resonance in photoproduction from the neutron
= +1 Baryon resonance in photoproduction from the neutronNakano, Takashi*; Ahn, D. S.*; Ahn, J. K.*; Akimune, Hidetoshi*; Asano, Yoshihiro; Chang, W. C.*; Date, S.*; Ejiri, Hiroyasu*; Fujimura, Hisako*; Fujiwara, Mamoru; et al.
Physical Review Letters, 91(1), p.012002_1 - 012002_4, 2003/07
Times Cited Count:1007 Percentile:99.86(Physics, Multidisciplinary)no abstracts in English
; Kawai, Masataka *
JNC TN9410 2001-002, 355 Pages, 2000/12
Durability in lithium of the capsule material which will be used for corrosion test of heat resisting metals was examined and the corrosion test procedure was confirmed. The tests were carried out from 500 C till maximum 1200
C till maximum 1200 C. Heating time per a test was 100 hr, and the capsule and the corrosion test specimens were observed after each test. The results were as follows. (1)The capsule material of Nb-1Zr was not remarkably attacked ti11 1200
C. Heating time per a test was 100 hr, and the capsule and the corrosion test specimens were observed after each test. The results were as follows. (1)The capsule material of Nb-1Zr was not remarkably attacked ti11 1200 C. (2)Welding part of capsule specimen was heat treated at 1200
C. (2)Welding part of capsule specimen was heat treated at 1200 C, 1 hr in argon to, improve corrosion resistance to lithium. At the surface of welding bead and heat affect zone of this part, grain boundaries were appeared from about 800
C, 1 hr in argon to, improve corrosion resistance to lithium. At the surface of welding bead and heat affect zone of this part, grain boundaries were appeared from about 800 C. It shows that the welding part is less corrosion resistance compared with base metal. But deep corrosion was not observed. (3)The capsule used for these tests was also observed, and obvious crack and lithium leak was not found. (4)From these tests it is confirmed that Nb-1Zr capsule is usable to lithium corrosion test until following time at each temperature. 800
C. It shows that the welding part is less corrosion resistance compared with base metal. But deep corrosion was not observed. (3)The capsule used for these tests was also observed, and obvious crack and lithium leak was not found. (4)From these tests it is confirmed that Nb-1Zr capsule is usable to lithium corrosion test until following time at each temperature. 800 C : 600hr or more 1000
C : 600hr or more 1000 C : 400hr or more 1200
C : 400hr or more 1200 C : 200hr or more (5)Corrosion of stainless steel include Ni was initiated at 700-800
C : 200hr or more (5)Corrosion of stainless steel include Ni was initiated at 700-800 C, and over 1000
C, and over 1000 C it became conspicuously.
C it became conspicuously.
Asada, Takashi; Kawai, Masataka *
JNC TN9410 2001-001, 153 Pages, 2000/12
Before the material corrosion tests in lithium, the reactions of lithium with air and ammonia that will be used for lithium cleaning were examined, and the results were as follows. (1)When lithium put into air, surface of lithium changes to black first but soon to white, and the white layer becomes gradually thick. The first black of lithium surface is nitride (Li N) and it changes to white lithium hydroxide (LiOH) by reaction with water in air, and it grows. The growth rate of the lithium hydroxide is about 1/10 in the desiccator (humidity of about 10%) compare with in air. (2)When lithium put into nitrogen, surface of lithium changes to black, and soon changes to brown and cracks at surface. At the same time with this cracking, weight of lithium piece increases and nitridation progresses respectively rapidly. This nitridation completed during 1-2days on lithium rod of 10mm in diameter, and increase in weight stopped. (3)Lithium melts in liquid ammonia and its melting rate is about 2-3 hour to lithium of 1g. The liquid ammonia after lithium melting showed dark brown.
N) and it changes to white lithium hydroxide (LiOH) by reaction with water in air, and it grows. The growth rate of the lithium hydroxide is about 1/10 in the desiccator (humidity of about 10%) compare with in air. (2)When lithium put into nitrogen, surface of lithium changes to black, and soon changes to brown and cracks at surface. At the same time with this cracking, weight of lithium piece increases and nitridation progresses respectively rapidly. This nitridation completed during 1-2days on lithium rod of 10mm in diameter, and increase in weight stopped. (3)Lithium melts in liquid ammonia and its melting rate is about 2-3 hour to lithium of 1g. The liquid ammonia after lithium melting showed dark brown.
; Kano, Shigeki; Tachi, Yoshiaki; Kawai, Masataka *
JNC TN9410 2000-013, 89 Pages, 2000/09
Lithium is one of goodcoolants because of high boiling point (1317 C), small specific gravity (0.47 at 600
C), small specific gravity (0.47 at 600 C) and large specific heat (1cal/g/
C) and large specific heat (1cal/g/ C). Therefore if lithium will be used in fast reactor for coolant, the heat efficiency of reactor will largely increase. Here the fundamental properties of lithium and the results of examination on chemical reaction, combustion and extinction are shown. These examinations were also carried out on sodium to compare with lithium. The differences between both are that lithium reacts more moderately with water, not explosive, and is not combustible but after ignition burns at higher temperature and longer.
C). Therefore if lithium will be used in fast reactor for coolant, the heat efficiency of reactor will largely increase. Here the fundamental properties of lithium and the results of examination on chemical reaction, combustion and extinction are shown. These examinations were also carried out on sodium to compare with lithium. The differences between both are that lithium reacts more moderately with water, not explosive, and is not combustible but after ignition burns at higher temperature and longer.