Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nakajima, Kenji; Kawakita, Yukinobu; Ito, Shinichi*; Abe, Jun*; Aizawa, Kazuya; Aoki, Hiroyuki; Endo, Hitoshi*; Fujita, Masaki*; Funakoshi, Kenichi*; Gong, W.*; et al.
Quantum Beam Science (Internet), 1(3), p.9_1 - 9_59, 2017/12
The neutron instruments suite, installed at the spallation neutron source of the Materials and Life Science Experimental Facility (MLF) at the Japan Proton Accelerator Research Complex (J-PARC), is reviewed. MLF has 23 neutron beam ports and 21 instruments are in operation for user programs or are under commissioning. A unique and challenging instrumental suite in MLF has been realized via combination of a high-performance neutron source, optimized for neutron scattering, and unique instruments using cutting-edge technologies. All instruments are/will serve in world-leading investigations in a broad range of fields, from fundamental physics to industrial applications. In this review, overviews, characteristic features, and typical applications of the individual instruments are mentioned.
Omori, Kazuaki; Hasegawa, Takashi; Munemoto, Takashi; Masuda, Kaoru*; Aosai, Daisuke*; Inui, Michiharu*; Iwatsuki, Teruki
JAEA-Data/Code 2014-019, 121 Pages, 2014/12
Japan Atomic Energy Agency has been investigating the groundwater chemistry on excavating the underground facilities as part of the Mizunami Underground research Laboratory (MIU) Project at Mizunami City, Gifu Prefecture, Japan. In this report, we compiled data obtained by geochemical research for groundwater at the MIU in the fiscal year 2013.
Omori, Kazuaki; Shingu, Shinya*; Masuda, Kaoru*; Aosai, Daisuke*; Inui, Michiharu*; Iwatsuki, Teruki
JAEA-Data/Code 2013-024, 284 Pages, 2014/03
Japan Atomic Energy Agency has been investigating the groundwater chemistry on excavating the underground facilities as part of the Mizunami Underground research Laboratory (MIU) Project at Mizunami City, Gifu Prefecture, Japan. In this report, we compiled data of groundwater chemistry obtained at the MIU in the fiscal year 2012.
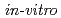 experimental results to model
experimental results to model 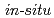 experiments; Bio-denitrification under geological disposal conditions
experiments; Bio-denitrification under geological disposal conditionsMasuda, Kaoru*; Murakami, Hiroshi*; Kurimoto, Noritaka*; Kato, Osamu*; Kato, Ko*; Honda, Akira
SpringerPlus (Internet), 2, p.339_1 - 339_13, 2013/07
Times Cited Count:2 Percentile:5.55(Multidisciplinary Sciences)Some of the low level radioactive wastes from reprocessing of spent nuclear fuels contain nitrates. Nitrates can be present in the form of soluble salts and be reduced by microorganisms. In this study, 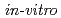 experiments of the nitrate reduction reaction were conducted using model organic materials purported to exist in underground conditions relevant to geological disposal. A reaction model was developed and verified by running simulations against data obtained from
experiments of the nitrate reduction reaction were conducted using model organic materials purported to exist in underground conditions relevant to geological disposal. A reaction model was developed and verified by running simulations against data obtained from 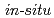 experiments using actual groundwaters and microorganisms. The simulation showed a good correlation with the experimental data and contributes to the understanding of microbially mediated denitrification in geological disposal systems.
experiments using actual groundwaters and microorganisms. The simulation showed a good correlation with the experimental data and contributes to the understanding of microbially mediated denitrification in geological disposal systems.
Omori, Kazuaki; Shingu, Shinya; Hagiwara, Hiroki; Masuda, Kaoru*; Iizuka, Masatoshi*; Inui, Michiharu*; Iwatsuki, Teruki
JAEA-Data/Code 2013-001, 330 Pages, 2013/05
Japan Atomic Energy Agency has been investigated the groundwater chemistry on excavating the underground facilities as part of the Mizunami Underground Research Laboratory (MIU) Project at Mizunami, Gifu, Japan. This report compiles data of the groundwater chemistry obtained at MIU in the fiscal year 2011. In terms of ensuring traceability of data, basic information (e.g. sampling location, sampling date, sampling method, analytical method) and methodology for quality control is described.
Shingu, Shinya; Hagiwara, Hiroki; Masuda, Kaoru*; Iizuka, Masatoshi*; Inui, Michiharu*; Mizuno, Takashi
JAEA-Data/Code 2012-003, 50 Pages, 2012/06
Japan Atomic Energy Agency has been investigated the groundwater chemistry on excavating the underground facilities as part of the Mizunami Underground Research Laboratory (MIU) Project at Mizunami City, Gifu Prefecture, Japan. This report compiles data set of the groundwater chemistry obtained at MIU in the fiscal year 2010. In terms of ensuring traceability of data, basic information (e.g. sampling location, sampling date, sampling method, analytical method) and methodology for quality control is described.
Kameyama, Hideo*; Sakurai, Makoto*; Masuda, Akiyuki*; Fukui, Tomoaki*; Onuki, Kaoru; Kubo, Shinji; Imai, Yoshiyuki
Suiso Enerugi Shisutemu, 37(1), p.3 - 10, 2012/03
The technical present status of hydrogen production process using IS thermochemical cycle was introduced. This process is experimentally investigated in Japan, U.S.A., Germany, France, Italy, India, China and Korea. Japan Atomic Energy Agency succeeded in consecutive hydrogen production as a proof examination. The trend of the research and development about the reactions, separation technology, device materials and the process equipment were reported. Thermochemical ammonia production cycle was also introduced. This new cycle is named ISN cycle which was modified from IS cycle in order to produce ammonia from water and nitrogen.
Honda, Akira; Masuda, Kaoru*; Tateishi, Tsuyoshi*; Kato, Osamu*; Inoue, Hiroyuki*
Zairyo To Kankyo, 60(12), p.541 - 552, 2011/12
Immersion tests and rest potential measurements under hyper-alkaline and high sodium nitrate concentrations were conducted to elucidate and improve model predictions of chemical interactions between carbon steel and nitrate ion in high concentrations of nitrate salt. The modified model can estimate the tendency of time dependent variation of chemical species and of rest potentials.
Honda, Akira; Masuda, Kaoru*; Imakita, Tsuyoshi*; Kato, Osamu*; Nishimura, Tsutomu*
Zairyo To Kankyo, 58(5), p.182 - 189, 2009/05
Interaction between carbon steel and nitrate was modeled using the mixed potential concept. Carbon steel was selected as an example of metal components in the repository of radioactive waste. The nitrate reduction accompanied by the corrosion of carbon steel was modeled as a reaction series such as nitrate - nitrite - ammonia. The sum of the current of the reaction series of nitrate - nitrite - ammonia and that of water reduction was assumed to balance with the corrosion current of carbon steel. The input parameters for this kinetic model were determined by electrochemical measurement and immersion test. The results of the immersion test can be interpreted by the analyses of the model.
Honda, Akira; Kato, Takashi; Tateishi, Tsuyoshi*; Imakita, Tsuyoshi*; Masuda, Kaoru*; Kato, Osamu*; Nishimura, Tsutomu*
Zairyo To Kankyo, 55(10), p.458 - 465, 2006/10
Migration of radioactive material can be affected by the redox condition and the concentration of ligands in the repository of radioactive waste. It is possible that radioactive waste contains nitrate which can affect the migration behavior of radioactive nuclides by both changing the redox condition of the environment and acting as a ligand. On the other hand, several researchers observed the reduction of nitrate ions in ammonia due to the iron. Ammonia has a potential to ligand for radioactive nuclides. Nitrate can also affect the rate of hydrogen gas evolution accompanied by metal corrosion through changing the rest potential of metal by its oxidizing nature. Carbon steel was, therefore, immersed in an aqueous solution of sodium nitrate in a closed system for observing both the chemical interaction between metal and nitrate, and the effect of nitrate on the hydrogen gas evolution rate. The experimental pH range of the solution was 10.0-13.5 which corresponds to the pH range of pore fluid of cementitious material. The cathodic current density shows a Tafel equation type potential dependency in the aqueous solution containing nitrate or nitrite. In spite of the acceleration of cathodic reaction due to the existence of nitrate, the corrosion rates of carbon steel were not accelerated in the nitrate solutions. This fact suggests that the system is controlled by the anodic reaction. The nitrate reduction accompanied by the corrosion of carbon steel is considered to be a series reaction such as nitrate nitrite ammonia. The nitrate reduction reaction compete with the water reduction reaction within the anodic controlled condition, therefore nitrate strongly reduced the hydrogen evolution rate. The generation rates of ammonia were independent of the concentration of nitrate.
Honda, Akira; Masuda, Kaoru*; Kato, Osamu*; Nishimura, Tsutomu*; Tateishi, Tsuyoshi*; Imakita, Tsuyoshi*
JNC TN8400 2005-023, 40 Pages, 2005/09
Uranium and Plutonium are planed to be recovered from spent fuel by the reprocessing in Japan. PUREX method is internationally dominant among the commercial reprocessing plants. PUREX method has been also employed in Japan. The low level liquid waste from PUREX process would contain NO3- as forms of soluble salts, if the special process for decomposing NO3- were not adopted. The nitrate is possibly brought within the repository for TRU waste. The specie of NO3- is an oxidizing agent which can be reduced to NO2- and NH3/NH4+ by the coexistence of reducing materials such as metals.In order to estimate the safety of the repository for TRU waste, the impacts of nitrate on the disposal system for TRU waste have to be estimated. Especially, NH3 can elevate the solubility and reduce Rd value through the formation of ammine complexes. The quantitative information of chemical evolution of nitrate is necessary for evaluating the impact of nitrate and the chemical species arising from nitrate on the safety of the repository of TRU waste. The evolution of chemical form of NO3- by the reducing reaction accompanied with metal corrosion was experimentally examined. The reduction of NO3- was considered to be a serial reaction, that is, NO3- - NO2- - NH3. The rate equations of cathodic reactions (water reduction and reduction of nitrate and nitrite) were experimentally determined through the electrochemical measurements. The rate equation of metal dissolution (anodic reaction) which must be balanced to the cathodic reactions in charge transfer is determined from the results of immersion tests without nitrate. The combination of the rate equations forms an assessment model of chemical evolution of NO3-. The model provided the interpretation of the results of immersion tests with nitrate.
Masuda, Kaoru*; Kato, Takashi; Honda, Akira
JNC TN8400 2005-021, 19 Pages, 2005/09
Uranium and Plutonium are planed to be recovered from spent fuel by the reprocessing in Japan. PUREX method is internationally dominant among the commercial reprocessing plants. PUREX method has been also employed in Japan. The low level liquid waste from PUREX process would contain NO
 as forms of soluble salts, if the special process for decomposing NO
as forms of soluble salts, if the special process for decomposing NO
 were not adopted. The nitrate is possibly brought within the repository for TRU waste. The generation of H
were not adopted. The nitrate is possibly brought within the repository for TRU waste. The generation of H gas and the reduction of NO
gas and the reduction of NO
 due to metal corrosion and the generation of N
due to metal corrosion and the generation of N gas due to the microbiological reactions in repository for group3 waste were estimated. The uncertainty on the hydraulic conductivity of cementitious materials, corrosion rates, and microbiological reactions are reflected to the analyses through setting the multiple cases. The analysis results suggested followings. (1) The maximum concentrations of both NO
gas due to the microbiological reactions in repository for group3 waste were estimated. The uncertainty on the hydraulic conductivity of cementitious materials, corrosion rates, and microbiological reactions are reflected to the analyses through setting the multiple cases. The analysis results suggested followings. (1) The maximum concentrations of both NO
 and NH
and NH were given in the case where the hydraulic conductivity of cementitious material was assumed to be low (
were given in the case where the hydraulic conductivity of cementitious material was assumed to be low ( 5.0
5.0 10
10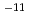 m/s) and the corrosion rate was constant at the initial value (0.1 um/y). (2) The maximum concentration of NH
m/s) and the corrosion rate was constant at the initial value (0.1 um/y). (2) The maximum concentration of NH was estimated to be 0.8 mol/dm
was estimated to be 0.8 mol/dm . (3) The impact of microbiological reaction (denitrifying reaction) was negligible in terms of yield of NO
. (3) The impact of microbiological reaction (denitrifying reaction) was negligible in terms of yield of NO
 and NH
and NH because the proportion of denitrified NO
because the proportion of denitrified NO
 was low. (4) The existence of NO
was low. (4) The existence of NO
 was strongly reduced the evolution of H
was strongly reduced the evolution of H gas because the nitrate reduction and nitrite reduction dominate the cathodic reactions. (5) The N
gas because the nitrate reduction and nitrite reduction dominate the cathodic reactions. (5) The N gas evolution rate due to the denitrifying reaction by microorganisms dominated the total gas evolution rate, if time dependent reduction of corrosion rate was employed. However, the H
gas evolution rate due to the denitrifying reaction by microorganisms dominated the total gas evolution rate, if time dependent reduction of corrosion rate was employed. However, the H gas evolution rate in the case is similar to those of N
gas evolution rate in the case is similar to those of N gas due to the denitrifying reaction mentioned above, if time dependent reduction of corrosion rate was neglected
gas due to the denitrifying reaction mentioned above, if time dependent reduction of corrosion rate was neglected
Wada, Ryutaro*; Nishimura, Tsutomu*; Masuda, Kaoru*; Fujiwara, Kazuo*; Imakita, Tsuyoshi*; Tateishi, Tsuyoshi*
JNC TJ8400 2004-017, 71 Pages, 2004/02
Research on changes of nitrate by interactions with metals under the wastes disposal environment containing TRU nuclide
Wada, Ryutaro*; Nishimura, Tsutomu*; Masuda, Kaoru*; Fujiwara, Kazuo*; Imakita, Tsuyoshi*; Tateishi, Tsuyoshi*
JNC TJ8400 2004-016, 194 Pages, 2004/02
Research on Changes of Nitrate by Chemical Interactions with Metals under the wastes Disposal Environment Contaioning TRU Nucride
Nagase, Yoshiyuki*; Masuda, Kaoru*; Wada, Ryutaro*; Yamamoto, Ichiro*; Tomioka, Osamu; Meguro, Yoshihiro; Fukuzato, Ryuichi*
Proceedings of 2nd International Symposium on Supercritical Fluid Technology for Energy and Environment Applications (Super Green 2003), p.254 - 257, 2004/00
no abstracts in English
Wada, Ryutaro*; Nishimura, Tsutomu*; Masuda, Kaoru*; Fujiwara, Kazuo*; Imakita, Tsuyoshi*; Tateishi, Tsuyoshi*
JNC TJ8400 2003-080, 153 Pages, 2003/02
There exists the waste including a nitrate ion as a salt in the TRU waste materials. This nitrate ion can be transferred to the nitrite ion and/or ammonia by reducing materials such as metals in the waste disposal environment, and has the possibility to affect on the disposal environment and nuclide transfer parameters.Therefore, electrochemical tests were conducted to evaluate the reaction rate parameters of the nitrate ion and metals under the low oxygen environment. The long-term reaction test using the glass-seal vessel was also conducted to grasp precisely the nitrate ion transition reaction rate and the gas generation rate caused by the reaction of metal and the nitrate ion coexist solution. (1) Reaction rate constants under various environments were obtained performing the potentiostatic holding tests with the parameters of the solution pH, temperature, and the nitrate and nitrite ion concentrations. The formula of the nitrate ion transition reaction rate was also examined based on these obtained data. (2) Conducting the immersion tests under the environment of the low oxygen and high-pH rainfall underground water site, the long-term reaction rate data were obtained on the reaction products (ammonia, hydrogen gas etc.) of metals (carbon steel, stainless steel and zircaloy etc.) with nitrate ion. The tests under the same conditions as in the past were also conducted to evaluate the test accuracy and error range of the long-term reaction test with the glass-seal vessels.
Wada, Ryutaro*; Nishimura, Tsutomu*; Masuda, Kaoru*; Fujiwara, Kazuo*; Imakita, Tsuyoshi*; Tateishi, Tsuyoshi*
JNC TJ8400 2003-079, 252 Pages, 2003/02
There exists the waste including a nitrate ion as a salt in the TRU waste materials. This nitrate ion can be transferred to the nitrite ion and/or ammonia by reducing materials such as metals in the waste disposal environment, and has the possibility to affect on the disposal environment and nuclide transfer parameters. Therefore, electrochemical tests were conducted to evaluate the reaction rate parameters of the nitrate ion and metals under the low oxygen environment. The long-term reaction test using the glass-seal vessel was also conducted to grasp precisely the nitrate ion transition reaction rate and the gas generation rate caused by the reaction of metal and the nitrate ion coexist solution. (1)}Reaction rate constants under various environments were obtained performing the potentiostatic holding tests with the parameters of the solution pH, temperature, and the nitrate and nitrite ion concentrations. The formula of the nitrate ion transition reaction rate was also examined based on these obtained data. (2) Conducting the immersion tests under the environment of the low oxygen and high-pH rainfall underground water site, the long-term reaction rate data were obtained on the reaction products (ammonia, hydrogen gas etc.) of metals (carbon steel, stainless steel and zircaloy etc.) with nitrate ion. The tests under the same conditions as in the past were also conducted to evaluate the test accuracy and error range of the long-term reaction test with the glass-seal vessels.
Wada, Ryutaro*; Nishimura, Tsutomu*; Masuda, Kaoru*; Fujiwara, Kazuo*; Imakita, Tsuyoshi*; Tateishi, Tsuyoshi*
JNC TJ8400 2003-077, 156 Pages, 2002/02
Some TRU wastes contain nitrate ions as salt.The nitrate ions might transform into NO2- and NH3, etc. in the disposal site environment because of reducing agent such as metals, possibly changing disposal site environment or affecting nuclide migration parameters.Therefore, we investigated of chemical interaction between NO3- and metals in a low oxygen environment that corresponds to the disposal site environment.
Wada, Ryutaro*; Nishimura, Tsutomu*; Masuda, Kaoru*; Fujiwara, Kazuo*; Imakita, Tsuyoshi*; Tateishi, Tsuyoshi*
JNC TJ8400 2003-076, 300 Pages, 2002/02
Some TRU wastes contain nitrate ions as salt.The nitrate ions might transform into NO2- and NH3, etc. in the disposal site environment because of reducing agent such as metals, possibly changing disposal site environment or affecting nuclide migration parameters. Therefore, we investigated of chemical interaction between NO3- and metals in a low oxygen environment that corresponds to the disposal site environment.
Masuda, Kaoru*; Murakami, Hiroshi*; Kato, Osamu*; Kato, Ko*; Kurimoto, Yoshitaka; Honda, Akira
no journal, ,
A model of nitrate reduction reaction due to microbial activity was developed as a part of an estimation system for the impacts of nitrate on the safety of a geological disposal system for radioactive wastes. Microbial reaction model, which incorporates a relation between nitrate reduction and consumption of organic materials, has been developed on the basis of data available in the literature and experimental results. The model can interpret the experimental results which were acquired using real groundwater.
Wada, Ryutaro*; Yamamoto, Seiichi*; Masuda, Kaoru*; Meguro, Yoshihiro; Tomioka, Osamu; Yamamoto, Ichiro*
no journal, ,
no abstracts in English